Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study
- PMID: 21353695
- PMCID: PMC3090685
- DOI: 10.1016/S0140-6736(10)62344-6
Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study
Abstract
Background: Bone metastases are a major burden in men with advanced prostate cancer. We compared denosumab, a human monoclonal antibody against RANKL, with zoledronic acid for prevention of skeletal-related events in men with bone metastases from castration-resistant prostate cancer.
Methods: In this phase 3 study, men with castration-resistant prostate cancer and no previous exposure to intravenous bisphosphonate were enrolled from 342 centres in 39 countries. An interactive voice response system was used to assign patients (1:1 ratio), according to a computer-generated randomisation sequence, to receive 120 mg subcutaneous denosumab plus intravenous placebo, or 4 mg intravenous zoledronic acid plus subcutaneous placebo, every 4 weeks until the primary analysis cutoff date. Randomisation was stratified by previous skeletal-related event, prostate-specific antigen concentration, and chemotherapy for prostate cancer within 6 weeks before randomisation. Supplemental calcium and vitamin D were strongly recommended. Patients, study staff, and investigators were masked to treatment assignment. The primary endpoint was time to first on-study skeletal-related event (pathological fracture, radiation therapy, surgery to bone, or spinal cord compression), and was assessed for non-inferiority. The same outcome was further assessed for superiority as a secondary endpoint. Efficacy analysis was by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00321620, and has been completed.
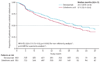
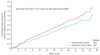
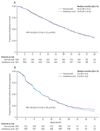
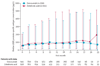
Findings: 1904 patients were randomised, of whom 950 assigned to denosumab and 951 assigned to receive zoledronic acid were eligible for the efficacy analysis. Median duration on study at primary analysis cutoff date was 12·2 months (IQR 5·9-18·5) for patients on denosumab and 11·2 months (IQR 5·6-17·4) for those on zoledronic acid. Median time to first on-study skeletal-related event was 20·7 months (95% CI 18·8-24·9) with denosumab compared with 17·1 months (15·0-19·4) with zoledronic acid (hazard ratio 0·82, 95% CI 0·71-0·95; p = 0·0002 for non-inferiority; p = 0·008 for superiority). Adverse events were recorded in 916 patients (97%) on denosumab and 918 patients (97%) on zoledronic acid, and serious adverse events were recorded in 594 patients (63%) on denosumab and 568 patients (60%) on zoledronic acid. More events of hypocalcaemia occurred in the denosumab group (121 [13%]) than in the zoledronic acid group (55 [6%]; p<0·0001). Osteonecrosis of the jaw occurred infrequently (22 [2%] vs 12 [1%]; p = 0·09).
Interpretation: Denosumab was better than zoledronic acid for prevention of skeletal-related events, and potentially represents a novel treatment option in men with bone metastases from castration-resistant prostate cancer.
Funding: Amgen.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
KF has received consultancy fees and travel support from Amgen for this study and from Novartis; participated in speakers’ bureaux and advisory boards for Amgen and Novartis; and provided expert testimony for Amgen. MC has received consultancy fees from Amgen for this study and for other agents in development, and from Novartis; and received research funding from Amgen. MS has received consultancy fees from Amgen; and participated in sponsored clinical research with Amgen and Novartis. RDam has received research funding from Amgen and the Center of Research in Urology Sergio Aguinaga (CEPUSA). JB has received travel support and payment for lectures from Amgen and Novartis; JB has received payment for membership of advisory boards from Amgen, Novartis, and GlaxoSmithKline; and JB and JB’s institution have received consultancy fees from Amgen. LK has received consultancy fees, travel support, and honoraria for lectures and development of educational presentations from Amgen; and LK’s institution has received research funding from Amgen. PM has received research funding from Amgen; and been a board member and principal investigator for, and received travel support from, Amgen. NS has received consultancy fees and travel support from Amgen for this study; and received research funding and honoraria from Amgen. MR has received travel support and honoraria for membership of advisory boards and lectures from Amgen; and MR’s institution has received research funding from Amgen for this study. HW, Q J, ST, RDan, and CG are employees of Amgen, and have received stock or stock options from Amgen.
Figures

Comment in
-
Unravelling the role of denosumab in prostate cancer.Lancet. 2011 Mar 5;377(9768):785-6. doi: 10.1016/S0140-6736(11)60100-1. Epub 2011 Feb 25. Lancet. 2011. PMID: 21353698 No abstract available.
-
Denosumab in bone-metastatic prostate cancer: known effects on skeletal-related events but unknown effects on quality of life.Asian J Androl. 2011 Jul;13(4):612-3. doi: 10.1038/aja.2011.33. Epub 2011 Apr 18. Asian J Androl. 2011. PMID: 21499282 Free PMC article. No abstract available.
-
Re: Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study.J Urol. 2011 Dec;186(6):2254-5. doi: 10.1016/j.juro.2011.08.108. Epub 2011 Oct 22. J Urol. 2011. PMID: 22078585 No abstract available.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. - PubMed
-
- Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–2342. - PubMed
-
- Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. - PubMed
-
- Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

