Cytoprotective protein C pathways and implications for stroke and neurological disorders
- PMID: 21353711
- PMCID: PMC3491752
- DOI: 10.1016/j.tins.2011.01.005
Cytoprotective protein C pathways and implications for stroke and neurological disorders
Abstract
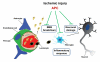
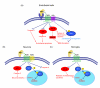
Recent studies indicate that single-action-single-target agents are unlikely to cure CNS disorders sharing a pathogenic triad consisting of vascular damage, neuronal injury/neurodegeneration and neuroinflammation. Here we focus on a recent example of a multiple-action-multiple-target approach for CNS disorders based on newly discovered biological properties of activated protein C (APC), an endogenous plasma protease with antithrombotic, cytoprotective and anti-inflammatory activities in the CNS. We propose that APC-mediated signaling through the protease activated receptor-1 (PAR1) can favorably regulate multiple pathways within the neurovascular unit in non-neuronal cells and neurons during acute or chronic CNS insults, leading to stabilization of the blood-brain barrier (BBB), neuroprotection and control of neuroinflammation. Although much remains to be understood regarding the biology of APC, preclinical studies suggest that APC has promising applications as disease-modifying therapy for ischemic stroke and other neuropathologies whose underlying pathology involves deficits in the vasculo-neuronal-inflammatory triad.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

