The Notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary
- PMID: 21354131
- PMCID: PMC3077720
- DOI: 10.1016/j.ydbio.2011.02.018
The Notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary
Abstract
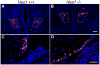
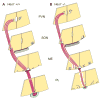
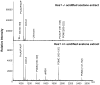
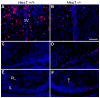
Proper development of the hypothalamic-pituitary axis requires precise neuronal signaling to establish a network that regulates homeostasis. The developing hypothalamus and pituitary utilize similar signaling pathways for differentiation in embryonic development. The Notch signaling effector gene Hes1 is present in the developing hypothalamus and pituitary and is required for proper formation of the pituitary, which contains axons of arginine vasopressin (AVP) neurons from the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON). We hypothesized that Hes1 is necessary for the generation, placement and projection of AVP neurons. We found that Hes1 null mice show no significant difference in cell proliferation or death in the developing diencephalon at embryonic day 10.5 (e10.5) or e11.5. By e16.5, AVP cell bodies are formed in the SON and PVN, but are abnormally placed, suggesting that Hes1 may be necessary for the migration of AVP neurons. GAD67 immunoreactivity is ectopically expressed in Hes1 null mice, which may contribute to cell body misplacement. Additionally, at e18.5 Hes1 null mice show continued misplacement of AVP cell bodies in the PVN and SON and additionally exhibit abnormal axonal projection. Using mass spectrometry to characterize peptide content, we found that Hes1 null pituitaries have aberrant somatostatin (SS) peptide, which correlates with abnormal SS cells in the pituitary and misplaced SS axon tracts at e18.5. Our results indicate that Notch signaling facilitates the migration and guidance of hypothalamic neurons, as well as neuropeptide content.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Akimoto M, Nishimaki T, Arai Y, Uchinuma E, Yamauchi H, Kameda Y. Hes1 Regulates Formations of the Hypophyseal Pars Tuberalis and the Hypothalamus. Cell Tissue Res. 2010;340:509–521. - PubMed
-
- Altman J, Bayer SA. Development of the Diencephalon in the Rat. IV. Quantitative Study of the Time of Origin of Neurons and the Internuclear Chronological Gradients in the Thalamus. J Comp Neurol. 1979;188:455–471. - PubMed
-
- Altman J, Bayer SA. Development of the Diencephalon in the Rat. I. Autoradiographic Study of the Time of Origin and Settling Patterns of Neurons of the Hypothalamus. J Comp Neurol. 1978;182:945–971. - PubMed
-
- Bayer SA, Altman J. Development of the Preoptic Area: Time and Site of Origin, Migratory Routes, and Settling Patterns of its Neurons. J Comp Neurol. 1987;265:65–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

