Removal of lactate dehydrogenase-elevating virus from human-in-mouse breast tumor xenografts by cell-sorting
- PMID: 21354210
- PMCID: PMC3086718
- DOI: 10.1016/j.jviromet.2011.02.015
Removal of lactate dehydrogenase-elevating virus from human-in-mouse breast tumor xenografts by cell-sorting
Abstract
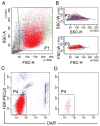
Lactate dehydrogenase-elevating virus (LDV) can infect transplantable mouse tumors or xenograft tumors in mice through LDV-contaminated mouse biological materials, such as Matrigel, or through mice infected with LDV. LDV infects specifically mouse macrophages and alters immune system and tumor phenotype. The traditional approaches to remove LDV from tumor cells, by transplanting tumors into rats or culturing tumor cells in vitro, are inefficient, labor-intensive and time-consuming. Furthermore, these approaches are not feasible for primary tumor cells that cannot survive tissue culture conditions or that may change phenotype in rats. This study reports that fluorescence-activated cell sorting (FACS) is a simple and efficient approach for purifying living primary human breast tumor cells from LDV(+) mouse stromal cells, which can be completed in a few hours. When purified from Matrigel contaminated LDV(+) tumors, sorted human breast tumor cells, as well as tumors grown from sorted cells, were shown to be LDV-free, as tested by PCR. The results demonstrate that cell sorting is effective, much faster and less likely to alter tumor cell phenotype than traditional methods for removing LDV from xenograft models. This approach may also be used to remove other rodent-specific viruses from models derived from distinct tissues or species with sortable markers, where virus does not replicate in the cells to be purified.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

References
-
- Infectious disease of mice and rats, Institute for Laboratory Animal Resources ed. National Academy Press; Washington, D.C:
-
- Anderson GW, Even C, Rowland RR, Palmer GA, Harty JT, Plagemann PG. C58 and AKR mice of all ages develop motor neuron disease after lactate dehydrogenase-elevating virus infection but only if antiviral immune responses are blocked by chemical or genetic means or as a result of old age. J Neurovirol. 1995;1:244–52. - PubMed
-
- Baker DG. Natural Pathogens of laboratory Animals: Their effects on research. ASM Press; Washington, D.C: 2003.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

