Differential protein expression throughout the life cycle of Trypanosoma congolense, a major parasite of cattle in Africa
- PMID: 21354217
- PMCID: PMC3820035
- DOI: 10.1016/j.molbiopara.2011.02.009
Differential protein expression throughout the life cycle of Trypanosoma congolense, a major parasite of cattle in Africa
Abstract
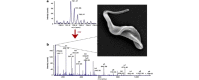
Trypanosoma congolense is an important pathogen of livestock in Africa. To study protein expression throughout the T. congolense life cycle, we used culture-derived parasites of each of the three main insect stages and bloodstream stage parasites isolated from infected mice, to perform differential protein expression analysis. Three complete biological replicates of all four life cycle stages were produced from T. congolense IL3000, a cloned parasite that is amenable to culture of major life cycle stages in vitro. Cellular proteins from each life cycle stage were trypsin digested and the resulting peptides were labeled with isobaric tags for relative and absolute quantification (iTRAQ). The peptides were then analyzed by tandem mass spectrometry (MS/MS). This method was used to identify and relatively quantify proteins from the different life cycle stages in the same experiment. A search of the Wellcome Trust's Sanger Institute's semi-annotated T. congolense database was performed using the MS/MS fragmentation data to identify the corresponding source proteins. A total of 2088 unique protein sequences were identified, representing 23% of the ∼9000 proteins predicted for the T. congolense proteome. The 1291 most confidently identified proteins were prioritized for further study. Of these, 784 yielded annotated hits while 501 were described as "hypothetical proteins". Six proteins showed no significant sequence similarity to any known proteins (from any species) and thus represent new, previously uncharacterized T. congolense proteins. Of particular interest among the remainder are several membrane molecules that showed drastic differential expression, including, not surprisingly, the well-studied variant surface glycoproteins (VSGs), invariant surface glycoproteins (ISGs) 65 and 75, congolense epimastigote specific protein (CESP), the surface protease GP63, an amino acid transporter, a pteridine transporter and a haptoglobin-hemoglobin receptor. Several of these surface disposed proteins are of functional interest as they are necessary for survival of the parasites.
Crown Copyright © 2011. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Swallow B.M. Impacts of trypanosomosis on African agriculture. Paper presented at the International Scientific Council for Trypanosomosis Research and Control; Maputo, Mozambique, September 29–October 4; 1997.
-
- Programme Against African Trypanosomiasis. Tsetse and Trypanosomiasis Information. PAAT 2007. Vol. 30, Part 2.
-
- Turner M.J. The biochemistry of the surface antigens of the African trypanosomes. Br Med Bull. 1985;41:137–143. - PubMed
-
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985;41:105–114. - PubMed
-
- Brun R., Schönenberger M. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 1979;36(3):289–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

