Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism
- PMID: 21354229
- PMCID: PMC3197749
- DOI: 10.1016/j.jhep.2011.02.011
Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism
Abstract
Background & aims: Hepatitis C virus (HCV) infection affects 3% of the world population and is the leading cause of chronic liver disease worldwide. Current standard of care is effective in only 50% of the patients, poorly tolerated, and associated with significant side effects and viral resistance. Recently, our group and others demonstrated that the HCV lifecycle is critically dependent on host lipid metabolism and that its production is metabolically modulated.
Methods: The JFH1/Huh7.5.1 full lifecycle model of HCV was used to study the antiviral effects of naringenin on viral replication, assembly, and production. Activation of PPARα was elucidated using GAL4-PPARα fusion reporters, PPRE reporters, qRT-PCR, and metabolic studies. Metabolic results were confirmed in primary human hepatocytes.
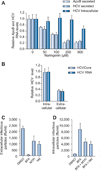
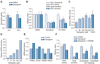
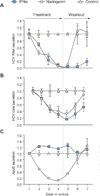
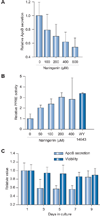
Results: We demonstrate that the grapefruit flavonoid naringenin dose-dependently inhibits HCV production without affecting intracellular levels of the viral RNA or protein. We show that naringenin blocks the assembly of intracellular infectious viral particles, upstream of viral egress. This antiviral effect is mediated in part by the activation of PPARα, leading to a decrease in VLDL production without causing hepatic lipid accumulation in Huh7.5.1 cells and primary human hepatocytes. Long-term treatment with naringenin leads to a rapid 1.4 log reduction in HCV, similar to 1000U of interferon. During the washout period, HCV levels returned to normal, consistent with our proposed mechanism of action.
Conclusions: The data demonstrates that naringenin is a non-toxic assembly inhibitor of HCV and that other PPARα agonists play a similar role in blocking viral production. The combination of naringenin with STAT-C agents could potentially bring a rapid reduction in HCV levels during the early treatment phase, an outcome associated with sustained virological response.
Copyright © 2011 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. - PubMed
-
- Stribling R, Sussman N, Vierling JM. Treatment of hepatitis C infection. Gastroenterol Clin North Am. 2006;35:463–486. - PubMed
-
- Serfaty L, Andreani T, Giral P, Carbonell N, Chazouilléres O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

