Alpha-fetoprotein-thymidine kinase-luciferase knockin mice: a novel model for dual modality longitudinal imaging of tumorigenesis in liver
- PMID: 21354236
- PMCID: PMC3465678
- DOI: 10.1016/j.jhep.2010.10.020
Alpha-fetoprotein-thymidine kinase-luciferase knockin mice: a novel model for dual modality longitudinal imaging of tumorigenesis in liver
Abstract
Background & aims: Hepatocellular carcinoma (HCC) is frequently a lethal disease and one of the few malignancies that is still increasing in incidence around the world. Better animal models are highly desired to investigate the molecular basis of HCC and to develop novel therapeutic strategies. Alpha-fetoprotein (Afp) gene is expressed in fetal liver, silenced soon after birth, and highly re-expressed in hepatocellular carcinomas (HCC). We aimed to take advantage of the dramatic re-expression of the Afp gene in HCC to develop a hepatocarcinogenesis reporter (HCR) mouse model for dual-modality, longitudinal in vivo imaging of liver tumor development, and progression.
Methods: Knock in mice were established by placing a thymidinekinase (tk)-luciferase (luc) reporter gene cassette under the transcriptional control of the endogenous Afp promoter. DEN, a liver carcinogen, was used to induce liver tumors, which was monitored by both luc-based bioluminescent (BL) and tk-based positron emission tomography (PET) imaging.
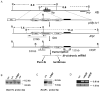
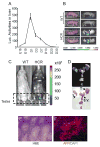
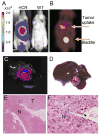
Results: The expression profile of luc was identical to that of the endogenous Afp gene during development. As early as 2 months after the exposure to DEN, BLI revealed multifocal signals in the liver, long before the appearance of histologically apparent neoplastic lesions. By 6 months, BL and PET dual imaging showed strong signals in malignant HCC. By serendipity, a strong BL signal was also detected in adult testes, a previously unknown site of Afp expression.
Conclusions: The HCR model enables longitudinal monitoring of liver tumor development and progression, providing a powerful tool in developing chemoprevention and therapeutic strategies for HCC.
Copyright © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures


Comment in
-
Focus.J Hepatol. 2011 Jul;55(1):1-2. doi: 10.1016/j.jhep.2011.03.001. Epub 2011 Mar 22. J Hepatol. 2011. PMID: 21396971 No abstract available.
References
-
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002 Aug;31(4):339–346. - PubMed
-
- Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003 Aug 11;22(33):5093–5107. - PubMed
-
- Varela M, Sanchez W, Bruix J, Gores GJ. Hepatocellular carcinoma in the setting of liver transplantation. Liver Transpl. 2006 Jul;12(7):1028–1036. - PubMed
-
- Ahmad I, Sansom OJ, Leung HY. Advances in mouse models of prostate cancer 3. Expert Rev Mol Med. 2008;10:e16. - PubMed
-
- Kim CF, Jackson EL, Kirsch DG, Grimm J, Shaw AT, Lane K, et al. Mouse models of human non-small-cell lung cancer: raising the bar. Cold Spring Harb Symp Quant Biol. 2005;70:241–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

