Catalytic and inhibitor binding properties of zebrafish monoamine oxidase (zMAO): comparisons with human MAO A and MAO B
- PMID: 21354322
- PMCID: PMC3071876
- DOI: 10.1016/j.cbpb.2011.02.002
Catalytic and inhibitor binding properties of zebrafish monoamine oxidase (zMAO): comparisons with human MAO A and MAO B
Erratum in
- Comp Biochem Physiol B Biochem Mol Biol. 2011 Oct;160(2-3):122
Abstract
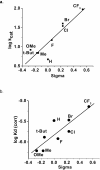
A comparative investigation of substrate specificity and inhibitor binding properties of recombinant zebrafish (Danio rerio) monoamine oxidase (zMAO) with those of recombinant human monoamine oxidases A and B (hMAO A and hMAO B) is presented. zMAO oxidizes the neurotransmitter amines (serotonin, dopamine and tyramine) with k(cat) values that exceed those of hMAO A or of hMAO B. The enzyme is competitively inhibited by hMAO A selective reversible inhibitors with the exception of d-amphetamine where uncompetitive inhibition is exhibited. The enzyme is unreactive with most MAO B-specific reversible inhibitors with the exception of chlorostyrylcaffeine. zMAO catalyzes the oxidation of para-substituted benzylamine analogs exhibiting (D)k(cat) and (D)(k(cat)/K(m)) values ranging from 2 to 8. Structure-activity correlations show a dependence of log k(cat) with the electronic factor σ(p) with a ρ value of +1.55±0.34; a value close to that for hMAO A but not with MAO B. zMAO differs from hMAO A or hMAO B in benzylamine analog binding correlations where an electronic effect (ρ=+1.29±0.31) is observed. These data demonstrate zMAO exhibits functional properties similar to hMAO A as well as exhibits its own unique behavior. These results should be useful for studies of MAO function in zebrafish models of human disease states.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Andreeva NI, Golovina SM, Mashkovskii MD. A new antidepressant tetrindole II. Experimental investigation of tetrindole tolerability. J Med Pharm Chem. 1992;26:11–12.
-
- Anichtchik O, Sallinen V, Peitsaro N, Panula P. Distinct structure and activity of monoamine oxidase in the brain of zebrafish (Danio rerio) J Comp. Neurol. 2006;498:593–610. - PubMed
-
- Binda C, Newton-Vinson P, Hubalek F, Edmondson DE, Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002;9:22–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

