Descending facilitatory pathways from the rostroventromedial medulla mediate naloxone-precipitated withdrawal in morphine-dependent rats
- PMID: 21354865
- PMCID: PMC4028695
- DOI: 10.1016/j.jpain.2010.12.007
Descending facilitatory pathways from the rostroventromedial medulla mediate naloxone-precipitated withdrawal in morphine-dependent rats
Abstract
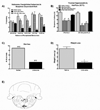
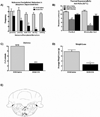
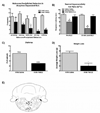
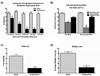
Opioids produce analgesic effects, and extended use can produce physical dependence in both humans and animals. Dependence to opiates can be demonstrated by either termination of drug administration or through precipitation of the withdrawal syndrome by opiate antagonists. Key features of the opiate withdrawal syndrome include hyperalgesia, anxiety, and autonomic signs such as diarrhea. The rostral ventromedial medulla (RVM) plays an important role in the modulation of pain and for this reason, may influence withdrawal-induced hyperalgesia. The mechanisms that drive opiate withdrawal-induced hyperalgesia have not been elucidated. Here, rats made dependent upon morphine received naloxone to precipitate withdrawal. RVM microinjection of lidocaine, kynurenic acid (excitatory amino acid antagonist) or YM022 (CCK2 receptor antagonist) blocked withdrawal-induced hyperalgesia. Additionally, these treatments reduced both somatic and autonomic signs of naloxone-induced withdrawal. Spinal application of ondansetron, a 5HT3 receptor antagonist thought to ultimately be engaged by descending pain facilitatory drive, also blocked hyperalgesia and somatic and autonomic features of the withdrawal syndrome. These results indicate that the RVM plays a critical role in mediating components of opioid withdrawal that may contribute to opioid dependence.
Perspective: Manipulations targeting these descending pathways from the RVM may diminish the consequences of prolonged opioid administration-induced dependence and be useful adjunct strategies in reducing the risk of opioid addiction.
Copyright © 2011 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance.J Neurosci. 2005 Jan 12;25(2):409-16. doi: 10.1523/JNEUROSCI.4054-04.2005. J Neurosci. 2005. PMID: 15647484 Free PMC article.
-
Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla.J Neurosci. 1991 May;11(5):1433-9. doi: 10.1523/JNEUROSCI.11-05-01433.1991. J Neurosci. 1991. PMID: 2027054 Free PMC article.
-
Reduction of opioid withdrawal symptoms and opioid-induced hyperalgesia by subcutaneous sumatriptan reveals central neuromodulation.J Pain. 2025 Aug;33:105456. doi: 10.1016/j.jpain.2025.105456. Epub 2025 Jun 4. J Pain. 2025. PMID: 40480471
-
Neurophysiological response properties of medullary pain-control neurons following chronic treatment with morphine or oxycodone: modulation by acute ketamine.J Neurophysiol. 2020 Sep 1;124(3):790-801. doi: 10.1152/jn.00343.2020. Epub 2020 Aug 5. J Neurophysiol. 2020. PMID: 32755331
-
Brain Reward Circuits in Morphine Addiction.Mol Cells. 2016 Sep;39(9):645-53. doi: 10.14348/molcells.2016.0137. Epub 2016 Aug 9. Mol Cells. 2016. PMID: 27506251 Free PMC article. Review.
Cited by
-
The perception and endogenous modulation of pain.Scientifica (Cairo). 2012;2012:561761. doi: 10.6064/2012/561761. Epub 2012 Dec 25. Scientifica (Cairo). 2012. PMID: 24278716 Free PMC article. Review.
-
The BTBR Mouse, Sociability, and Reduced Glutamate Release: A Role for Endogenous Dynorphin?Neurochem Res. 2017 Aug;42(8):2435-2436. doi: 10.1007/s11064-017-2231-y. Epub 2017 Mar 16. Neurochem Res. 2017. PMID: 28303500 No abstract available.
-
Divergent profiles of fentanyl withdrawal and associated pain in mice and rats.Pharmacol Biochem Behav. 2021 Jan;200:173077. doi: 10.1016/j.pbb.2020.173077. Epub 2020 Dec 11. Pharmacol Biochem Behav. 2021. PMID: 33316293 Free PMC article.
-
Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain.Pain. 2016 Feb;157(2):387-398. doi: 10.1097/j.pain.0000000000000330. Pain. 2016. PMID: 26313406 Free PMC article.
-
Single-chain Fragment variable antibody targeting cholecystokinin-B receptor for pain reduction.Neurobiol Pain. 2021 Jul 15;10:100067. doi: 10.1016/j.ynpai.2021.100067. eCollection 2021 Aug-Dec. Neurobiol Pain. 2021. PMID: 34458647 Free PMC article.
References
-
- Ali NM. Hyperalgesic response in a patient receiving high concentrations of spinal morphine. Anesthesiology. 1986;65:449. - PubMed
-
- Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. - PubMed
-
- Aricioglu-Kartal F, Kayir H, Tayfun Uzbay I. Effects of harman and harmine on naloxone-precipitated withdrawal syndrome in morphine-dependent rats. Life Sci. 2003;73:2363–2371. - PubMed
-
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

