Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer
- PMID: 21355020
- PMCID: PMC3073446
- DOI: 10.1634/theoncologist.2010-0317
Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer
Abstract
Purpose: B-Raf(V600E) may play a role in the progression from papillary thyroid cancer to anaplastic thyroid cancer (ATC). We tested the effects of a highly selective B-Raf(V600E) inhibitor, PLX4720, on proliferation, migration, and invasion both in human thyroid cancer cell lines (8505c(B-RafV600E) and TPC-1(RET/PTC-1 and wild-type B-Raf)) and in primary human normal thyroid (NT) follicular cells engineered with or without B-Raf(V600E).
Experimental design: Large-scale genotyping analysis by mass spectrometry was performed in order to analyze >900 gene mutations. Cell proliferation and migration/invasion were performed upon PLX4720 treatment in 8505c, TPC-1, and NT cells. Orthotopic implantation of either 8505c or TPC-1 cells into the thyroid of severe combined immunodeficient mice was performed. Gene validations were performed by quantitative polymerase chain reaction and immunohistochemistry.
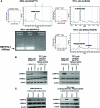
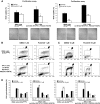
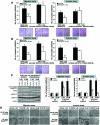
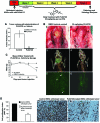
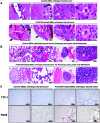
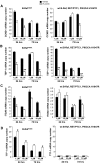
Results: We found that PLX4720 reduced in vitro cell proliferation and migration and invasion of 8505c cells, causing early downregulation of genes involved in tumor progression. PLX4720-treated NT cells overexpressing B-Raf(V600E) (heterozygous wild-type B-Raf/B-Raf(V600E)) showed significantly lower cell proliferation, migration, and invasion. PLX4720 treatment did not block cell invasion in TPC-1 cells with wild-type B-Raf, which showed very low and delayed in vivo tumor growth. In vivo, PLX4720 treatment of 8505c orthotopic thyroid tumors inhibited tumor aggressiveness and significantly upregulated the thyroid differentiation markers thyroid transcription factor 1 and paired box gene 8.
Conclusions: Here, we have shown that PLX4720 preferentially inhibits migration and invasion of B-Raf(V600E) thyroid cancer cells and tumor aggressiveness. Normal thyroid cells were generated to be heterozygous for wild-type B-Raf/B-Raf(V600E), mimicking the condition found in most human thyroid cancers. PLX4720 was effective in reducing cell proliferation, migration, and invasion in this heterozygous model. PLX4720 therapy should be tested and considered for a phase I study for the treatment of patients with B-Raf(V600E) ATC.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
Similar articles
-
The BRAF-inhibitor PLX4720 inhibits CXCL8 secretion in BRAFV600E mutated and normal thyroid cells: a further anti-cancer effect of BRAF-inhibitors.Sci Rep. 2019 Mar 13;9(1):4390. doi: 10.1038/s41598-019-40818-w. Sci Rep. 2019. PMID: 30867499 Free PMC article.
-
B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression.Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10649-54. doi: 10.1073/pnas.1004934107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498063 Free PMC article.
-
A Combinatorial Strategy for Targeting BRAF V600E-Mutant Cancers with BRAFV600E Inhibitor (PLX4720) and Tyrosine Kinase Inhibitor (Ponatinib).Clin Cancer Res. 2020 Apr 15;26(8):2022-2036. doi: 10.1158/1078-0432.CCR-19-1606. Epub 2020 Jan 14. Clin Cancer Res. 2020. PMID: 31937621 Free PMC article.
-
Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E).Biochim Biophys Acta. 2009 Apr;1795(2):152-61. doi: 10.1016/j.bbcan.2009.01.003. Epub 2009 Jan 31. Biochim Biophys Acta. 2009. PMID: 19356676 Free PMC article. Review.
-
Orthotopic mouse models for the preclinical and translational study of targeted therapies against metastatic human thyroid carcinoma with BRAF(V600E) or wild-type BRAF.Oncogene. 2014 Nov 20;33(47):5397-404. doi: 10.1038/onc.2013.544. Epub 2013 Dec 23. Oncogene. 2014. PMID: 24362526 Free PMC article. Review.
Cited by
-
Modified TI-RADS Coupled with BRAFV600E Enhances Diagnostic Efficiency in Papillary Thyroid Carcinoma: Prospective Study.Int J Gen Med. 2024 Jul 10;17:3015-3025. doi: 10.2147/IJGM.S456820. eCollection 2024. Int J Gen Med. 2024. PMID: 39006910 Free PMC article.
-
FOXM1 is a molecular determinant of the mitogenic and invasive phenotype of anaplastic thyroid carcinoma.Endocr Relat Cancer. 2012 Sep 21;19(5):695-710. doi: 10.1530/ERC-12-0031. Print 2012 Oct. Endocr Relat Cancer. 2012. PMID: 22919068 Free PMC article.
-
The BRAF-inhibitor PLX4720 inhibits CXCL8 secretion in BRAFV600E mutated and normal thyroid cells: a further anti-cancer effect of BRAF-inhibitors.Sci Rep. 2019 Mar 13;9(1):4390. doi: 10.1038/s41598-019-40818-w. Sci Rep. 2019. PMID: 30867499 Free PMC article.
-
Clinical outcome, role of BRAF(V600E), and molecular pathways in papillary thyroid microcarcinoma: is it an indolent cancer or an early stage of papillary thyroid cancer?Front Endocrinol (Lausanne). 2012 Feb 27;3:33. doi: 10.3389/fendo.2012.00033. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22649416 Free PMC article.
-
Mouse models of follicular and papillary thyroid cancer progression.Front Endocrinol (Lausanne). 2012 Jan 10;2:119. doi: 10.3389/fendo.2011.00119. eCollection 2011. Front Endocrinol (Lausanne). 2012. PMID: 22654848 Free PMC article.
References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology 2007. [accessed September 2009]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
-
- Pacini F. Where do we stand with targeted therapy of refractory thyroid cancer?—utility of RECIST criteria. Thyroid. 2008;18:279–280. - PubMed
-
- Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. - PubMed
-
- Santoro M, Melillo RM, Carlomagno F, et al. Molecular mechanisms of RET activation in human cancer. Ann N Y Acad Sci. 2002;963:116–121. - PubMed
-
- Giordano TJ, Kuick R, Thomas DG, et al. Molecular classification of papillary thyroid carcinoma: Distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–6656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

