The dominant negative β isoform of the glucocorticoid receptor is uniquely expressed in erythroid cells expanded from polycythemia vera patients
- PMID: 21355091
- PMCID: PMC3138692
- DOI: 10.1182/blood-2010-07-296921
The dominant negative β isoform of the glucocorticoid receptor is uniquely expressed in erythroid cells expanded from polycythemia vera patients
Abstract
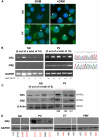
Glucocorticoid receptor (GR) agonists increase erythropoiesis in vivo and in vitro. To clarify the effect of the dominant negative GRβ isoform (unable to bind STAT-5) on erythropoiesis, erythroblast (EB) expansion cultures of mononuclear cells from 18 healthy (nondiseased) donors (NDs) and 16 patients with polycythemia vera (PV) were studied. GRβ was expressed in all PV EBs but only in EBs from 1 ND. The A3669G polymorphism, which stabilizes GRβ mRNA, had greater frequency in PV (55%; n = 22; P = .0028) and myelofibrosis (35%; n = 20) patients than in NDs (9%; n = 22) or patients with essential thrombocythemia (6%; n = 15). Dexamethasone stimulation of ND cultures increased the number of immature EBs characterized by low GATA1 and β-globin expression, but PV cultures generated great numbers of immature EBs with low levels of GATA1 and β-globin irrespective of dexamethasone stimulation. In ND EBs, STAT-5 was not phosphorylated after dexamethasone and erythropoietin treatment and did not form transcriptionally active complexes with GRα, whereas in PV EBs, STAT-5 was constitutively phosphorylated, but the formation of GR/STAT-5 complexes was prevented by expression of GRβ. These data indicate that GRβ expression and the presence of A3669G likely contribute to development of erythrocytosis in PV and provide a potential target for identification of novel therapeutic agents.
Figures


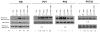


References
-
- Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976;295(17):913–916. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(8):1054–1061. - PubMed
-
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
-
- Constantinescu SN, Ghaffari S, Lodish HF. The erythropoietin receptor: structure, activation and intracellular signal transduction. Trends Endocrinol Metab. 1999;10(1):18–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

