Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus
- PMID: 21356043
- PMCID: PMC3052239
- DOI: 10.1186/1741-7007-9-14
Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus
Abstract
Background: Evc is essential for Indian Hedgehog (Hh) signalling in the cartilage growth plate. The gene encoding Evc2 is in close proximity in divergent orientation to Evc and mutations in both human genes lead to the chondrodysplasia Ellis-van Creveld syndrome.
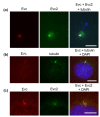
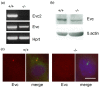
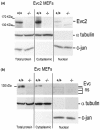
Results: Bioinformatic analysis reveals that the Evc and Evc2 genes arose through a duplication event early in metazoan evolution and were subsequently lost in arthropods and nematodes. Here we demonstrate that Evc2 is essential for Hh pathway activation in response to the Smo agonist purmorphamine. A yeast two-hybrid screen using Evc as bait identified Evc2 as an Evc binding partner and we confirmed the interaction by immunoprecipitation. We developed anti-Evc2 antibodies and show that Evc2 and Evc co-localize at the basal body and also on primary cilia. In transfected cells, basal body and cilia localization is observed when Evc and Evc2 constructs are co-transfected but not when either construct is transfected individually. We show that Evc and Evc2 are cilia transmembrane proteins, the C-terminus for both being intracellular and Evc2, but not Evc, having an extracellular portion. Furthermore, Evc is absent at the basal body in Evc2 null cells. Using Western blots of cytoplasmic and nuclear protein, we also demonstrate that full length Evc2 but not Evc, is located in the nucleus.
Conclusions: We demonstrate for the first time that Evc2 is a positive regulator of the Hh signalling pathway and that it is located at the basal body of primary cilia. We show that the presence of Evc and Evc2 at the basal body and cilia membrane is co-dependent. In addition, Evc2, but not Evc, is present in the cell nucleus suggesting movement of Evc2 between the cilium and nucleus.
Figures
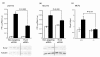



Similar articles
-
Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2.Cell Res. 2012 Nov;22(11):1593-604. doi: 10.1038/cr.2012.134. Epub 2012 Sep 18. Cell Res. 2012. PMID: 22986504 Free PMC article.
-
The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia.Hum Mol Genet. 2013 Jan 1;22(1):124-39. doi: 10.1093/hmg/dds409. Epub 2012 Oct 1. Hum Mol Genet. 2013. PMID: 23026747
-
Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands.Am J Med Genet C Semin Med Genet. 2009 Nov 15;151C(4):341-51. doi: 10.1002/ajmg.c.30226. Am J Med Genet C Semin Med Genet. 2009. PMID: 19876929 Review.
-
Role of primary cilia and Hedgehog signaling in craniofacial features of Ellis-van Creveld syndrome.Am J Med Genet C Semin Med Genet. 2022 Mar;190(1):36-46. doi: 10.1002/ajmg.c.31969. Epub 2022 Apr 8. Am J Med Genet C Semin Med Genet. 2022. PMID: 35393766 Review.
-
Specific variants in WDR35 cause a distinctive form of Ellis-van Creveld syndrome by disrupting the recruitment of the EvC complex and SMO into the cilium.Hum Mol Genet. 2015 Jul 15;24(14):4126-37. doi: 10.1093/hmg/ddv152. Epub 2015 Apr 23. Hum Mol Genet. 2015. PMID: 25908617 Free PMC article.
Cited by
-
Molecular and Cellular Pathogenesis of Ellis-van Creveld Syndrome: Lessons from Targeted and Natural Mutations in Animal Models.J Dev Biol. 2020 Oct 9;8(4):25. doi: 10.3390/jdb8040025. J Dev Biol. 2020. PMID: 33050204 Free PMC article. Review.
-
Transcriptome analysis of cervical cancer exosomes and detection of HPVE6*I transcripts in exosomal RNA.BMC Cancer. 2022 Feb 11;22(1):164. doi: 10.1186/s12885-022-09262-4. BMC Cancer. 2022. PMID: 35148692 Free PMC article.
-
Novel large deletion involving EVC and EVC2 in Ellis-van Creveld syndrome.Hum Genome Var. 2022 May 17;9(1):15. doi: 10.1038/s41439-022-00190-0. Hum Genome Var. 2022. PMID: 35581188 Free PMC article.
-
Expression of Evc2 in craniofacial tissues and craniofacial bone defects in Evc2 knockout mouse.Arch Oral Biol. 2016 Aug;68:142-52. doi: 10.1016/j.archoralbio.2016.05.002. Epub 2016 May 3. Arch Oral Biol. 2016. PMID: 27164562 Free PMC article.
-
Ellis-van Creveld syndrome novel pathogenic variant in the EVC2 gene a patient from Turkey.Clin Case Rep. 2021 Feb 14;9(4):1973-1976. doi: 10.1002/ccr3.3919. eCollection 2021 Apr. Clin Case Rep. 2021. PMID: 33936625 Free PMC article.
References
-
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S, Marino B, Dallapiccola B, Wright M, Meitinger T, Polymeropoulos MH, Goodship J. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24(3):283–286. doi: 10.1038/73508. - DOI - PubMed
-
- Ruiz-Perez VL, Tompson SW, Blair HJ, Espinoza-Valdez C, Lapunzina P, Silva EO, Hamel B, Gibbs JL, Young ID, Wright MJ, Goodship JA. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am J Hum Genet. 2003;72(3):728–732. doi: 10.1086/368063. - DOI - PMC - PubMed
-
- Genomic Reference Consortium GRCh37 human assembly. http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/index.shtml
-
- Genomic Reference Consortium Build 37 mouse assembly. http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/mouse/index.shtml
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

