Assessment of efficacy and impact on work productivity and attendance after a mandatory switch to generic second-generation antihistamines: results of a patient survey in Norway
- PMID: 21356091
- PMCID: PMC3053265
- DOI: 10.1186/1476-7961-9-5
Assessment of efficacy and impact on work productivity and attendance after a mandatory switch to generic second-generation antihistamines: results of a patient survey in Norway
Abstract
Background: In 2006, the Norwegian Medicines Agency mandated a switch from desloratadine, ebastine, or fexofenadine to cetirizine or loratadine in patients with allergic rhinitis (AR) or chronic urticaria (CU). In an online survey, patients whose medication was switched assessed the impact on efficacy, fatigue, and work productivity/attendance.
Methods: Allergy patients in Norway completed a 25-item online survey. Patients aged ≥ 18 years with AR or CU who were switched to cetirizine or loratadine from desloratadine, ebastine, or fexofenadine were included. Participants rated post-switch efficacy, fatigue, and effect on work productivity/attendance compared with their pre-switch medication. Patients also reported post-switch change in number of doctor visits required, total treatment cost, and whether they had switched or wanted to switch back to their previous medications.
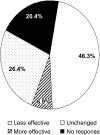
Results: Of 1920 patients invited, 493 responded and 409 of these were eligible. Previous antihistamines were desloratadine (78.4% of respondents), ebastine (16.0%), and fexofenadine (5.6%). Post-switch, 64.7% received cetirizine and 35.3% loratadine. Compared with previous therapy, cetirizine and loratadine were rated less effective by 46.3% of respondents; 28.7% reported increased fatigue; and 31.6% reported decreased work productivity with the generic agents. At the time of the survey, 26% of respondents had switched back to their previous medication.
Conclusions: This is the first survey to assess the impact on patient-reported outcomes of a mandated switch from prescription to generic antihistamines in Norway. The findings suggest that patient response to different antihistamines will vary and that treatment decisions should be individualized for optimal results.
Figures
References
-
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML. World Health Organization; GA(2)LEN; AllerGen et al.Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 Update (in collaboration with the World Health Organization, GA2LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. - DOI - PubMed
-
- Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, Grattan CE, Kapp A, Maurer M, Merk HF, Rogala B, Saini S, Sánchez-Borges M, Schmid-Grendelmeier P, Schünemann H, Staubach P, Vena GA, Wedi B. Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA2LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–1443. doi: 10.1111/j.1398-9995.2009.02178.x. - DOI - PubMed
-
- Sugiura K, Hirai S, Suzuki T, Usuda T, Kondo T, Azumi T, Masaki S, Yokoi T, Nitta Y, Kamiya S, Ando K, Mori T, Tomita Y. Evaluation of cetirizine hydrochloride-based therapeutic strategy for chronic urticaria. Nagoya J Med Sci. 2008;70:97–106. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials