Down-regulation of methylthioadenosine phosphorylase (MTAP) induces progression of hepatocellular carcinoma via accumulation of 5'-deoxy-5'-methylthioadenosine (MTA)
- PMID: 21356366
- PMCID: PMC3069916
- DOI: 10.1016/j.ajpath.2010.11.059
Down-regulation of methylthioadenosine phosphorylase (MTAP) induces progression of hepatocellular carcinoma via accumulation of 5'-deoxy-5'-methylthioadenosine (MTA)
Abstract
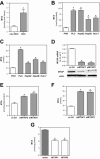
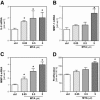
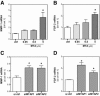
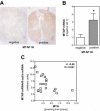
Recently, we have shown that down-regulation of methylthioadenosine phosphorylase (MTAP) in hepatocellular carcinoma (HCC) cells enhances the invasive potential and the resistance against cytokines. Here, we aimed at investigating the molecular mechanism underlying this tumor-promoting effect and expanded the analysis to a large series of human HCC tissues. Liquid chromatography tandem mass spectrometry revealed that reduced MTAP expression resulted in higher intra- and extracellular concentrations of 5'-deoxy-5'-methylthioadenosine (MTA) in cultivated HCC cells and, concordantly, higher levels of MTA in HCC tissue. MTA induced matrix metalloproteinase (MMP) and interleukin-8 transcription in HCC cells in vitro, accompanied by enhanced proliferation and activation of the transcription factor NFκB. In addition, MTA secreted by HCC cells induced expression of fibroblast growth factor-2 and MMP1 in stromal myofibroblasts. In human HCC tissues, MTAP mRNA correlated inversely with MTA levels, and immunohistochemical analysis of a tissue microarray of 140 human HCCs revealed that low MTAP protein expression correlated with advanced tumor stages. In conclusion, MTAP deficiency results in accumulation of MTA, which is associated with increased tumorigenicity. These data further indicate MTAP as a tumor suppressor in HCC, and MTA as a potential biomarker for HCC progression.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Shariff M.I.F., Cox I.J., Gomaa A.I., Khan S.A., Gedroyc W., Taylor-Robinson S.D. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353–367. - PubMed
-
- Hussain K., El-Serag H.B. Epidemiology, screening, diagnosis and treatment of hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2009;55:123–138. - PubMed
-
- Mendizabal M., Reddy K.R. Current management of hepatocellular carcinoma. Med Clin North Am. 2009;93:885–900. viii. - PubMed
-
- Yu M.C., Yuan J.M. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–S78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

