Albuminuria and glomerular damage in mice lacking the metabotropic glutamate receptor 1
- PMID: 21356376
- PMCID: PMC3070555
- DOI: 10.1016/j.ajpath.2010.11.050
Albuminuria and glomerular damage in mice lacking the metabotropic glutamate receptor 1
Abstract
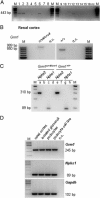
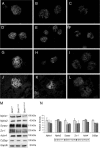
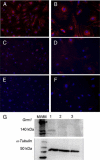
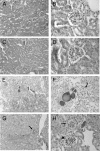
The metabotropic glutamate (mGlu) receptor 1 (GRM1) has been shown to play an important role in neuronal cells by triggering, through calcium release from intracellular stores, various signaling pathways that finally modulate neuron excitability, synaptic plasticity, and mechanisms of feedback regulation of neurotransmitter release. Herein, we show that Grm1 is expressed in glomerular podocytes and that a glomerular phenotype is exhibited by Grm1(crv4) mice carrying a spontaneous recessive inactivating mutation of the gene. Homozygous Grm1(crv4/crv4) and, to a lesser extent, heterozygous mice show albuminuria, podocyte foot process effacement, and reduced levels of nephrin and other proteins known to contribute to the maintenance of podocyte cell structure. Overall, the present data extend the role of mGlu1 receptor to the glomerular filtration barrier. The regulatory action of mGlu1 receptor in dendritic spine morphology and in the control of glutamate release is well acknowledged in neuronal cells. Analogously, we speculate that mGlu1 receptor may regulate foot process morphology and intercellular signaling in the podocyte.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Rastaldi M.P., Armelloni S., Berra S., Calvaresi N., Corbelli A., Giardino L.A., Li M., Wang G.Q., Fornasieri A., Villa A., Heikkila E., Soliymani R., Boucherot A., Cohen C.D., Kretzler M., Nitsche A., Ripamonti M., Malgaroli A., Pesaresi M., Forloni G.L., Schlondorff D., Holthofer H., D'Amico G. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J. 2006;20:976–978. - PubMed
-
- Giardino L., Armelloni S., Corbelli A., Mattinzoli D., Zennaro C., Guerrot D., Tourrel F., Ikehata M., Li M., Berra S., Carraro M., Messa P., Rastaldi M.P. Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol. 2009;20:1929–1940. - PMC - PubMed
-
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. - PubMed
-
- Fagni L., Chavis P., Ango F., Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

