M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis
- PMID: 21356378
- PMCID: PMC3069905
- DOI: 10.1016/j.ajpath.2010.11.065
M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis
Abstract
The etiopathogenesis of sarcoidosis, a systemic granulomatous disease, still remains obscure. A multitude of organs have been described to be affected in systemic sarcoidosis. Skeletal muscles may also be affected, leading to myalgia and weakness. A workup of the specific immune response with emphasis on the macrophage response is provided herein. Affected muscle tissue from seven patients with systemic sarcoidosis was analyzed and compared with that from seven patients with other myopathies containing macrophagocytic infiltration. Monocytes/macrophages and giant cells in granulomas of muscle tissue from patients with sarcoidosis show a status of alternative activation (M2) based on their expression of CD206, CD301, arginase-1, and suppressor of cytokine signaling-1 as a consequence of a functionally type 2 helper T cell (Th2)-biased cytokine profile. Significant fibrosis and up-regulation of CCL18 were associated with the M2 phenotype of macrophages. Conversely, up-regulated Th1 cytokines did not result in significant classical activation of macrophages (M1), with poor inducible nitric oxide synthase and cyclooxygenase-2 production. Giant cell formation was further associated with up-regulated expression of DNAX-activating protein of 12 kDa (DAP12; gene symbol TYROBP). Functionally, alternative activation of macrophages on the basis of a Th2-biased immune response may induce clinical symptoms and chronic evolution of neuromuscular sarcoidosis. This is the first characterization of Th2-mediated immune mechanisms in neuromuscular sarcoidosis suggesting that a specific macrophage activation status leading to myofibrosis may be a key event in the pathogenesis of this disease.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
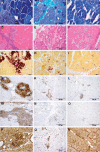




References
-
- Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., Holland S.M., Horsburgh R., Huitt G., Iademarco M.F., Iseman M., Olivier K., Ruoss S., von Reyn C.F., Wallace R.J., Jr, Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. - PubMed
-
- Morey S.S. American Thoracic Society issues consensus statement on sarcoidosis. Am Fam Physician. 2000;61:553–554. 556. - PubMed
-
- Parrish S., Turner J.F. Diagnosis of sarcoidosis. Dis Mon. 2009;55:693–703. - PubMed
-
- Lazarus A. Sarcoidosis: epidemiology, etiology, pathogenesis, and genetics. Dis Mon. 2009;55:649–660. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

