Regulation of lipid homeostasis by the bifunctional SREBF2-miR33a locus
- PMID: 21356514
- PMCID: PMC3062104
- DOI: 10.1016/j.cmet.2011.02.004
Regulation of lipid homeostasis by the bifunctional SREBF2-miR33a locus
Abstract
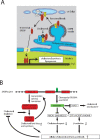
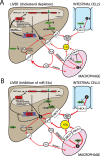
The sterol regulatory element-binding factor-2 (SREBF2) gene is a bifunctional locus encoding SREBP-2, a well-known transcriptional regulator of genes involved in cholesterol biosynthesis, and microRNA-33a, which has recently been shown to reduce expression of proteins involved in export of cholesterol and β-oxidation of fatty acids, thus adding an unexpected layer of complexity and fine-tuning to regulation of lipid homeostasis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
MicroRNAs regulating lipid metabolism in atherogenesis.Thromb Haemost. 2012 Apr;107(4):642-7. doi: 10.1160/TH11-10-0694. Epub 2012 Jan 25. Thromb Haemost. 2012. PMID: 22274626 Free PMC article. Review.
-
Methyl protodioscin increases ABCA1 expression and cholesterol efflux while inhibiting gene expressions for synthesis of cholesterol and triglycerides by suppressing SREBP transcription and microRNA 33a/b levels.Atherosclerosis. 2015 Apr;239(2):566-70. doi: 10.1016/j.atherosclerosis.2015.02.034. Epub 2015 Feb 23. Atherosclerosis. 2015. PMID: 25733328
-
SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression.J Lipid Res. 2016 Mar;57(3):410-21. doi: 10.1194/jlr.M064022. Epub 2015 Dec 18. J Lipid Res. 2016. PMID: 26685326 Free PMC article.
-
Enhanced Liver Regeneration After Partial Hepatectomy in Sterol Regulatory Element-Binding Protein (SREBP)-1c-Null Mice is Associated with Increased Hepatocellular Cholesterol Availability.Cell Physiol Biochem. 2018;47(2):784-799. doi: 10.1159/000490030. Epub 2018 May 22. Cell Physiol Biochem. 2018. PMID: 29807364
-
Endoplasmic reticulum stress and lipid dysregulation.Expert Rev Mol Med. 2011 Feb 3;13:e4. doi: 10.1017/S1462399410001742. Expert Rev Mol Med. 2011. PMID: 21288373 Review.
Cited by
-
MicroRNA-33a mediates the regulation of high mobility group AT-hook 2 gene (HMGA2) by thyroid transcription factor 1 (TTF-1/NKX2-1).J Biol Chem. 2013 Jun 7;288(23):16348-16360. doi: 10.1074/jbc.M113.474643. Epub 2013 Apr 26. J Biol Chem. 2013. PMID: 23625920 Free PMC article.
-
Consequences of exchanging carbohydrates for proteins in the cholesterol metabolism of mice fed a high-fat diet.PLoS One. 2012;7(11):e49058. doi: 10.1371/journal.pone.0049058. Epub 2012 Nov 6. PLoS One. 2012. PMID: 23139832 Free PMC article.
-
Beneficial effects of cherry consumption as a dietary intervention for metabolic, hepatic and vascular complications in type 2 diabetic rats.Cardiovasc Diabetol. 2018 Jul 20;17(1):104. doi: 10.1186/s12933-018-0744-6. Cardiovasc Diabetol. 2018. PMID: 30029691 Free PMC article.
-
Interaction effect between NAFLD severity and high carbohydrate diet on gut microbiome alteration and hepatic de novo lipogenesis.Gut Microbes. 2022 Jan-Dec;14(1):2078612. doi: 10.1080/19490976.2022.2078612. Gut Microbes. 2022. PMID: 35634707 Free PMC article.
-
A global view of aging and Alzheimer's pathogenesis-associated cell population dynamics and molecular signatures in human and mouse brains.Nat Genet. 2023 Dec;55(12):2104-2116. doi: 10.1038/s41588-023-01572-y. Epub 2023 Nov 30. Nat Genet. 2023. PMID: 38036784 Free PMC article.
References
-
- Brunham LR, Kastelein JJ, Hayden MR. ABCA1 gene mutations, HDL cholesterol levels, and risk of ischemic heart disease. JAMA. 2008;300:1997–1998. author reply 1998. - PubMed
-
- Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

