Phosphate incorporation during glycogen synthesis and Lafora disease
- PMID: 21356517
- PMCID: PMC3124772
- DOI: 10.1016/j.cmet.2011.01.017
Phosphate incorporation during glycogen synthesis and Lafora disease
Abstract
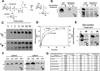
Glycogen is a branched polymer of glucose that serves as an energy store. Phosphate, a trace constituent of glycogen, has profound effects on glycogen structure, and phosphate hyperaccumulation is linked to Lafora disease, a fatal progressive myoclonus epilepsy that can be caused by mutations of laforin, a glycogen phosphatase. However, little is known about the metabolism of glycogen phosphate. We demonstrate here that the biosynthetic enzyme glycogen synthase, which normally adds glucose residues to glycogen, is capable of incorporating the β-phosphate of its substrate UDP-glucose at a rate of one phosphate per approximately 10,000 glucoses, in what may be considered a catalytic error. We show that the phosphate in glycogen is present as C2 and C3 phosphomonoesters. Since hyperphosphorylation of glycogen causes Lafora disease, phosphate removal by laforin may thus be considered a repair or damage control mechanism.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Glycogen synthase: an old enzyme with a new trick.Cell Metab. 2011 Mar 2;13(3):233-4. doi: 10.1016/j.cmet.2011.02.006. Cell Metab. 2011. PMID: 21356510
References
-
- Ball S, Guan HP, James M, Myers A, Keeling P, Mouille G, Buleon A, Colonna P, Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. - PubMed
-
- Blennow A, Nielsen TH, Baunsgaard L, Mikkelsen R, Engelsen SB. Starch phosphorylation: a new front line in starch research. Trends Plant Sci. 2002;7:445–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

