Rapid reconstitution of antibody responses following transplantation of purified allogeneic hematopoietic stem cells
- PMID: 21357265
- PMCID: PMC5516799
- DOI: 10.4049/jimmunol.1003674
Rapid reconstitution of antibody responses following transplantation of purified allogeneic hematopoietic stem cells
Abstract
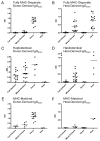
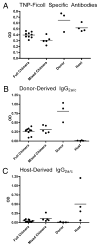
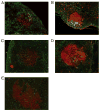
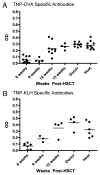
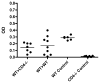
Allogeneic hematopoietic cell transplantation has broad clinical applications extending from the treatment of malignancies to induction of immunologic tolerance. However, adaptive cellular and humoral immunity frequently remain impaired posttransplantation. Here, recovery of T-dependent and T-independent Ab responses was evaluated in mice transplanted with purified hematopoietic stem cells (HSCs) devoid of the mature immune cells believed to hasten immune recovery. Mixed and full donor chimeras were created by conditioning recipients with sublethal or lethal irradiation, respectively, across different donor/host genetic disparities. By 6 wk posttransplantation, all animals demonstrated robust T-independent Ab responses, and all mixed chimeras and recipients of MHC-matched or haploidentical HSCs with a shared MHC haplotype had T-dependent Ab responses equivalent to those of untransplanted controls. Full chimeras that received fully MHC-disparate HSCs showed delayed T-dependent Ab responses that recovered by 12 wk. This delay occurred despite early reconstitution and proper migration to germinal centers of donor-derived T(follicular helper) (T(FH)) cells. Congenic transplants into T(FH)-deficient CD4(-/-) mice revealed restoration of T-dependent Ab responses by 6 wk, leading us to conclude that MHC disparity caused delay in humoral recovery. These findings, together with our previous studies, show that, contrary to the view that depletion of graft lymphocytes results in poor posttransplant immunity, elimination of immune-suppressing graft-versus-host reactions permits superior immune reconstitution. This study also provides insight into the regeneration of T(FH) cells and humoral immunity after allogeneic HSC transplantation.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


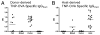
Similar articles
-
Transplantation of purified hematopoietic stem cells: requirements for overcoming the barriers of allogeneic engraftment.Biol Blood Marrow Transplant. 1996 Feb;2(1):3-14. Biol Blood Marrow Transplant. 1996. PMID: 9078349
-
Innate and adaptive immune responses are tolerized in chimeras prepared with nonmyeloablative conditioning.Transplantation. 2012 Mar 15;93(5):469-76. doi: 10.1097/TP.0b013e318242bddf. Transplantation. 2012. PMID: 22228418 Free PMC article.
-
Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation.Blood. 2005 Feb 15;105(4):1828-36. doi: 10.1182/blood-2004-08-3213. Epub 2004 Oct 19. Blood. 2005. PMID: 15494429
-
Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
-
Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells.Immunol Rev. 2000 Apr;174:260-79. doi: 10.1034/j.1600-0528.2002.017409.x. Immunol Rev. 2000. PMID: 10807522 Review.
References
-
- Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. - PubMed
-
- Witherspoon RP, Storb R, Ochs HD, Fluornoy N, Kopecky KJ, Sullivan KM, Deeg JH, Sosa R, Noel DR, Atkinson K, Thomas ED. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981;58:360–368. - PubMed
-
- Wagner JE, Thompson JS, Carter SL, Kernan NA Unrelated Donor Marrow Transplantation Trial. Effect of graft-versus-host disease pro-phylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

