Δ40 Isoform of p53 controls β-cell proliferation and glucose homeostasis in mice
- PMID: 21357466
- PMCID: PMC3064094
- DOI: 10.2337/db09-1379
Δ40 Isoform of p53 controls β-cell proliferation and glucose homeostasis in mice
Abstract
Objective: Investigating the dynamics of pancreatic β-cell mass is critical for developing strategies to treat both type 1 and type 2 diabetes. p53, a key regulator of the cell cycle and apoptosis, has mostly been a focus of investigation as a tumor suppressor. Although p53 alternative transcripts can modulate p53 activity, their functions are not fully understood. We hypothesized that β-cell proliferation and glucose homeostasis were controlled by Δ40p53, a p53 isoform lacking the transactivation domain of the full-length protein that modulates total p53 activity and regulates organ size and life span in mice.
Research design and methods: We phenotyped metabolic parameters in Δ40p53 transgenic (p44tg) mice and used quantitative RT-PCR, Western blotting, and immunohistochemistry to examine β-cell proliferation.
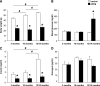
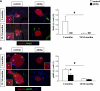
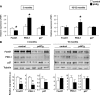
Results: Transgenic mice with an ectopic p53 gene encoding Δ40p53 developed hypoinsulinemia and glucose intolerance by 3 months of age, which worsened in older mice and led to overt diabetes and premature death from ∼14 months of age. Consistent with a dramatic decrease in β-cell mass and reduced β-cell proliferation, lower expression of cyclin D2 and pancreatic duodenal homeobox-1, two key regulators of proliferation, was observed, whereas expression of the cell cycle inhibitor p21, a p53 target gene, was increased.
Conclusions: These data indicate a significant and novel role for Δ40p53 in β-cell proliferation with implications for the development of age-dependent diabetes.
Figures





Similar articles
-
Delta40p53 controls the switch from pluripotency to differentiation by regulating IGF signaling in ESCs.Genes Dev. 2010 Nov 1;24(21):2408-19. doi: 10.1101/gad.1987810. Genes Dev. 2010. PMID: 21041409 Free PMC article.
-
Dominant effects of Δ40p53 on p53 function and melanoma cell fate.J Invest Dermatol. 2014 Mar;134(3):791-800. doi: 10.1038/jid.2013.391. Epub 2013 Sep 13. J Invest Dermatol. 2014. PMID: 24037342 Free PMC article.
-
[Effect of Δ40p53 isoform on enhancing the pro-apoptotic function of p53 in tumor cells].Zhonghua Zhong Liu Za Zhi. 2017 May 23;39(5):332-338. doi: 10.3760/cma.j.issn.0253-3766.2017.05.003. Zhonghua Zhong Liu Za Zhi. 2017. PMID: 28535648 Chinese.
-
p53 Isoforms in Cellular Senescence- and Ageing-Associated Biological and Physiological Functions.Int J Mol Sci. 2019 Nov 29;20(23):6023. doi: 10.3390/ijms20236023. Int J Mol Sci. 2019. PMID: 31795382 Free PMC article. Review.
-
Cellular and organismal ageing: Role of the p53 tumor suppressor protein in the induction of transient and terminal senescence.J Cell Biochem. 2007 Aug 15;101(6):1355-69. doi: 10.1002/jcb.21383. J Cell Biochem. 2007. PMID: 17471501 Review.
Cited by
-
The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis.Cells. 2022 Jun 19;11(12):1966. doi: 10.3390/cells11121966. Cells. 2022. PMID: 35741095 Free PMC article. Review.
-
Biological functions of p53 isoforms through evolution: lessons from animal and cellular models.Cell Death Differ. 2011 Dec;18(12):1815-24. doi: 10.1038/cdd.2011.120. Epub 2011 Sep 23. Cell Death Differ. 2011. PMID: 21941372 Free PMC article. Review.
-
Exploring the molecular mechanisms underlying α- and β-cell dysfunction in diabetes.Diabetol Int. 2017 Jun 29;8(3):248-256. doi: 10.1007/s13340-017-0327-x. eCollection 2017 Aug. Diabetol Int. 2017. PMID: 30603330 Free PMC article.
-
Control of metabolism by p53 - Cancer and beyond.Biochim Biophys Acta Rev Cancer. 2018 Aug;1870(1):32-42. doi: 10.1016/j.bbcan.2018.06.001. Epub 2018 Jun 5. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29883595 Free PMC article. Review.
-
The MDM2-p53-pyruvate carboxylase signalling axis couples mitochondrial metabolism to glucose-stimulated insulin secretion in pancreatic β-cells.Nat Commun. 2016 Jun 6;7:11740. doi: 10.1038/ncomms11740. Nat Commun. 2016. PMID: 27265727 Free PMC article.
References
-
- Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC. Increased islet beta cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia 2006;49:2689–2696 - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 - PubMed
-
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 2006;27:356–370 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG026094/AG/NIA NIH HHS/United States
- K99 DK090210/DK/NIDDK NIH HHS/United States
- R01-AG-026094/AG/NIA NIH HHS/United States
- R01-DK-67536/DK/NIDDK NIH HHS/United States
- R21 RR024905/RR/NCRR NIH HHS/United States
- R01 DK067536/DK/NIDDK NIH HHS/United States
- R01-DK-60581/DK/NIDDK NIH HHS/United States
- R01 DK060581/DK/NIDDK NIH HHS/United States
- R00 DK090210/DK/NIDDK NIH HHS/United States
- 1-K99-DK-090210-01/DK/NIDDK NIH HHS/United States
- RL9 EB008539/EB/NIBIB NIH HHS/United States
- R21-RR-024905/RR/NCRR NIH HHS/United States
- 1RL9-EB-008539-01/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

