Rapamycin treatment augments both protein ubiquitination and Akt activation in pressure-overloaded rat myocardium
- PMID: 21357504
- PMCID: PMC3094075
- DOI: 10.1152/ajpheart.00545.2010
Rapamycin treatment augments both protein ubiquitination and Akt activation in pressure-overloaded rat myocardium
Abstract
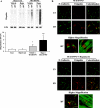
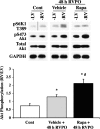
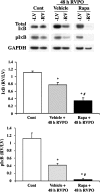
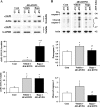
Ubiquitin-mediated protein degradation is necessary for both increased ventricular mass and survival signaling for compensated hypertrophy in pressure-overloaded (PO) myocardium. Another molecular keystone involved in the hypertrophic growth process is the mammalian target of rapamycin (mTOR), which forms two distinct functional complexes: mTORC1 that activates p70S6 kinase-1 to enhance protein synthesis and mTORC2 that activates Akt to promote cell survival. Independent studies in animal models show that rapamycin treatment that alters mTOR complexes also reduces hypertrophic growth and increases lifespan by an unknown mechanism. We tested whether the ubiquitin-mediated regulation of growth and survival in hypertrophic myocardium is linked to the mTOR pathway. For in vivo studies, right ventricle PO in rats was conducted by pulmonary artery banding; the normally loaded left ventricle served as an internal control. Rapamycin (0.75 mg/kg per day) or vehicle alone was administered intraperitoneally for 3 days or 2 wk. Immunoblot and immunofluorescence imaging showed that the level of ubiquitylated proteins in cardiomyocytes that increased following 48 h of PO was enhanced by rapamycin. Rapamycin pretreatment also significantly increased PO-induced Akt phosphorylation at S473, a finding confirmed in cardiomyocytes in vitro to be downstream of mTORC2. Analysis of prosurvival signaling in vivo showed that rapamycin increased PO-induced degradation of phosphorylated inhibitor of κB, enhanced expression of cellular inhibitor of apoptosis protein 1, and decreased active caspase-3. Long-term rapamycin treatment in 2-wk PO myocardium blunted hypertrophy, improved contractile function, and reduced caspase-3 and calpain activation. These data indicate potential cardioprotective benefits of rapamycin in PO hypertrophy.
Figures


Similar articles
-
Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension.Am J Respir Cell Mol Biol. 2013 May;48(5):568-77. doi: 10.1165/rcmb.2012-0429OC. Am J Respir Cell Mol Biol. 2013. PMID: 23470622 Free PMC article.
-
Rapamycin prevents thyroid hormone-induced cardiac hypertrophy.Endocrinology. 2007 Jul;148(7):3477-84. doi: 10.1210/en.2007-0099. Epub 2007 Mar 29. Endocrinology. 2007. PMID: 17395699
-
Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes.Adv Biol Regul. 2015 Jan;57:64-74. doi: 10.1016/j.jbior.2014.09.004. Epub 2014 Sep 18. Adv Biol Regul. 2015. PMID: 25442674
-
Reduced menin expression impairs rapamycin effects as evidenced by an increase in mTORC2 signaling and cell migration.Cell Commun Signal. 2018 Oct 1;16(1):64. doi: 10.1186/s12964-018-0278-2. Cell Commun Signal. 2018. PMID: 30285764 Free PMC article.
-
mTOR in growth and protection of hypertrophying myocardium.Cardiovasc Hematol Agents Med Chem. 2009 Jan;7(1):52-63. doi: 10.2174/187152509787047603. Cardiovasc Hematol Agents Med Chem. 2009. PMID: 19149544 Free PMC article. Review.
Cited by
-
Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling.Int J Mol Med. 2020 Jan;45(1):195-209. doi: 10.3892/ijmm.2019.4407. Epub 2019 Nov 13. Int J Mol Med. 2020. PMID: 31746373 Free PMC article.
-
Exercise Training Attenuates Right Ventricular Remodeling in Rats with Pulmonary Arterial Stenosis.Front Physiol. 2016 Dec 5;7:541. doi: 10.3389/fphys.2016.00541. eCollection 2016. Front Physiol. 2016. PMID: 27994552 Free PMC article.
-
Mammalian Target of Rapamycin Signaling Enhances Ovalbumin-Induced Neutrophilic Airway Inflammation by Promoting Th17 Cell Polarization in Murine Noneosinophilic Asthma Model.Pediatr Allergy Immunol Pulmonol. 2020 Mar;33(1):25-32. doi: 10.1089/ped.2019.1088. Pediatr Allergy Immunol Pulmonol. 2020. PMID: 33406024 Free PMC article.
-
mTOR Complexes Repress Hypertrophic Agonist-Stimulated Expression of Connective Tissue Growth Factor in Adult Cardiac Muscle Cells.J Cardiovasc Pharmacol. 2016 Feb;67(2):110-20. doi: 10.1097/FJC.0000000000000322. J Cardiovasc Pharmacol. 2016. PMID: 26371948 Free PMC article.
-
Okadaic acid induces Akt hyperphosphorylation and an oxidative stress-mediated cell death in serum starved SK-N-SH human neuroblastoma cells that are augmented by rapamycin.Neurosci Lett. 2012 Dec 7;531(2):74-9. doi: 10.1016/j.neulet.2012.10.052. Epub 2012 Nov 2. Neurosci Lett. 2012. PMID: 23127854 Free PMC article.
References
-
- Balasubramanian S, Mani S, Shiraishi H, Johnston RK, Yamane K, Willey CD, Cooper GT, Tuxworth WJ, Kuppuswamy D. Enhanced ubiquitination of cytoskeletal proteins in pressure overloaded myocardium is accompanied by changes in specific E3 ligases. J Mol Cell Cardiol 41: 669–679, 2006 - PubMed
-
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell 12: 487–502, 2007 - PubMed
-
- Boluyt MO, Li ZB, Loyd AM, Scalia AF, Cirrincione GM, Jackson RR. The mTOR/p70S6K signal transduction pathway plays a role in cardiac hypertrophy and influences expression of myosin heavy chain genes in vivo. Cardiovasc Drugs Ther 18: 257–267, 2004 - PubMed
-
- Cooper G, Satava RM. A method for producing reversible long-term pressure overload of the cat right ventricle. J Appl Physiol 37: 762–764, 1974 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

