β1 integrin is critical for the maintenance of antigen-specific CD4 T cells in the bone marrow but not long-term immunological memory
- PMID: 21357540
- PMCID: PMC3062718
- DOI: 10.4049/jimmunol.1003566
β1 integrin is critical for the maintenance of antigen-specific CD4 T cells in the bone marrow but not long-term immunological memory
Abstract
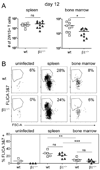
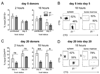
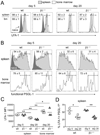
The long-term maintenance of memory CD4 T cells promotes protective immunity against future pathogen reinfection. As a site rich in survival cytokines, the bone marrow is proposed to be a critical niche for the survival of memory CD4 T cells. We demonstrate that endogenous, polyclonal Ag-specific CD4 T cells rapidly enter and are recovered long-term from the bone marrow following i.v. infection with Listeria monocytogenes. β(1) integrin-deficient CD4 T cells also populate the bone marrow early following an infection, but their numbers in this site rapidly decline. This decline was not caused by increased death of T cells lacking β(1) integrin but rather by reduced retention in the bone marrow after the primary immune response. The loss of memory CD4 T cells from the bone marrow does not lead to a loss of the predominant source of memory CD4 T cells in the spleen or the ability to mount a memory response. Thus, β(1) integrin-dependent maintenance of memory CD4 T cells in the bone marrow is not required for long-term CD4 T cell memory.
Figures



References
-
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. - PubMed
-
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. - PubMed
-
- Di Rosa F. T-lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol. Cell Biol. 2009;87:20–29. - PubMed
-
- Benner R, Meima F, van der Meulen GM. Antibody formation in mouse bone marrow. II. Evidence for a memory-dependent phenomenon. Cell. Immunol. 1974;13:95–106. - PubMed
-
- Price PW, Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCR Vβ repertoire. Eur. J. Immunol. 1999;29:1051–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

