Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus
- PMID: 21357542
- PMCID: PMC3159153
- DOI: 10.4049/jimmunol.1003057
Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus
Abstract
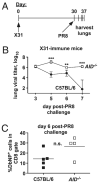
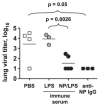
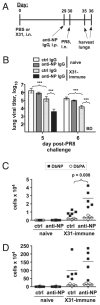
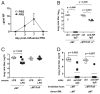
Influenza A virus causes recurring seasonal epidemics and occasional influenza pandemics. Because of changes in envelope glycoprotein Ags, neutralizing Abs induced by inactivated vaccines provide limited cross-protection against new viral serotypes. However, prior influenza infection induces heterosubtypic immunity that accelerates viral clearance of a second strain, even if the external proteins are distinct. In mice, cross-protection can also be elicited by systemic immunization with the highly conserved internal nucleoprotein (NP). Both T lymphocytes and Ab contribute to such cross-protection. In this paper, we demonstrate that anti-NP IgG specifically promoted influenza virus clearance in mice by using a mechanism involving both FcRs and CD8(+) cells. Furthermore, anti-NP IgG rescued poor heterosubtypic immunity in B cell-deficient mice, correlating with enhanced NP-specific CD8 T cell responses. Thus, Ab against this conserved Ag has potent antiviral activity both in naive and in influenza-immune subjects. Such antiviral activity was not seen when mice were vaccinated with another internal influenza protein, nonstructural 1. The high conservation of NP Ag and the known longevity of Ab responses suggest that anti-NP IgG may provide a critically needed component of a universal influenza vaccine.
Figures


Similar articles
-
Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance.J Virol. 2011 May;85(10):5027-35. doi: 10.1128/JVI.00150-11. Epub 2011 Mar 2. J Virol. 2011. PMID: 21367900 Free PMC article.
-
A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus.J Immunol. 2008 Sep 15;181(6):4168-76. doi: 10.4049/jimmunol.181.6.4168. J Immunol. 2008. PMID: 18768874 Free PMC article.
-
Diversifying T-cell responses: safeguarding against pandemic influenza with mosaic nucleoprotein.J Virol. 2025 Mar 18;99(3):e0086724. doi: 10.1128/jvi.00867-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898643 Free PMC article.
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Measuring Cellular Immunity to Influenza: Methods of Detection, Applications and Challenges.Vaccines (Basel). 2015 Apr 14;3(2):293-319. doi: 10.3390/vaccines3020293. Vaccines (Basel). 2015. PMID: 26343189 Free PMC article. Review.
Cited by
-
Cross-Protective Immune Responses Induced by Sequential Influenza Virus Infection and by Sequential Vaccination With Inactivated Influenza Vaccines.Front Immunol. 2018 Oct 9;9:2312. doi: 10.3389/fimmu.2018.02312. eCollection 2018. Front Immunol. 2018. PMID: 30356772 Free PMC article.
-
Antiviral Approaches against Influenza Virus.Clin Microbiol Rev. 2023 Mar 23;36(1):e0004022. doi: 10.1128/cmr.00040-22. Epub 2023 Jan 16. Clin Microbiol Rev. 2023. PMID: 36645300 Free PMC article. Review.
-
Influenza NP core and HA or M2e shell double-layered protein nanoparticles induce broad protection against divergent influenza A viruses.Nanomedicine. 2022 Feb;40:102479. doi: 10.1016/j.nano.2021.102479. Epub 2021 Nov 4. Nanomedicine. 2022. PMID: 34743020 Free PMC article.
-
Recent Progress in Recombinant Influenza Vaccine Development Toward Heterosubtypic Immune Response.Front Immunol. 2022 May 19;13:878943. doi: 10.3389/fimmu.2022.878943. eCollection 2022. Front Immunol. 2022. PMID: 35663997 Free PMC article. Review.
-
Influenza Vaccines: Successes and Continuing Challenges.J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S405-S419. doi: 10.1093/infdis/jiab269. J Infect Dis. 2021. PMID: 34590139 Free PMC article.
References
-
- Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, Zeng H, Tumpey TM, Katz JM. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol. Rev. 2008;225:68–84. - PubMed
-
- Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. - PubMed
-
- Whitley RJ, Monto AS. Seasonal and pandemic influenza preparedness: a global threat. J. Infect. Dis. 2006;194(Suppl. 2):S65–S69. - PubMed
-
- Du L, Zhou Y, Jiang S. Research and development of universal influenza vaccines. Microbes Infect. 2010;12:280–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

