Contrast-enhanced sonography depicts spontaneous ovarian cancer at early stages in a preclinical animal model
- PMID: 21357555
- PMCID: PMC3105598
- DOI: 10.7863/jum.2011.30.3.333
Contrast-enhanced sonography depicts spontaneous ovarian cancer at early stages in a preclinical animal model
Abstract
Objective: Our goal was to examine the feasibility of using laying hens, a preclinical model of human spontaneous ovarian cancer, in determining the kinetics of an ultrasound contrast agent indicative of ovarian tumor-associated neoangiogenesis in early-stage ovarian cancer.
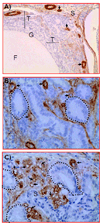
Methods: Three-year-old White Leghorn laying hens with decreased ovarian function were scanned before and after intravenous injection of a human serum albumin-perflutren contrast agent at a dose of 5 μL/kg body weight. Gray scale morphologic characteristics, Doppler indices, the arrival time, peak intensity, and wash-out of the contrast agent were recorded and archived on still images and video clips. Hens were euthanized thereafter; sonographic predictions were compared at gross examination; and ovarian tissues were collected. Archived clips were analyzed to determine contrast parameters and Doppler intensities of vessels. A time-intensity curve per hen was drawn, and the area under the curve was derived. Tumor types and the density of ovarian microvessels were determined by histologic examination and immunohistochemistry and compared to sonographic predictions.
Results: The contrast agent significantly (P < .05) enhanced the visualization of microvessels, which was confirmed by immunohistochemistry. Contrast parameters, including the time of wash-out and area under the curve, were significantly different (P < .05) between ovaries of normal hens and hens with ovarian cancer and correctly detected cancer at earlier stages than the time of peak intensity.
Conclusions: The laying hen may be a useful animal model for determining ovarian tumor-associated vascular kinetics diagnostic of early-stage ovarian cancer using a contrast agent. This model may also be useful for testing the efficacy of different contrast agents in a preclinical setting.
Figures





References
-
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. - PubMed
-
- Ramakrishnan S, Subramanian IV, Yokoyama Y, Geller M. Angiogenesis in normal and neoplastic ovaries. Angiogenesis. 2005;8:169–182. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

