Recognition and blocking of innate immunity cells by Candida albicans chitin
- PMID: 21357722
- PMCID: PMC3088140
- DOI: 10.1128/IAI.01282-10
Recognition and blocking of innate immunity cells by Candida albicans chitin
Abstract
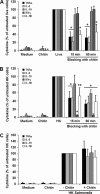
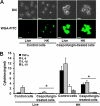
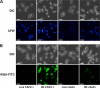
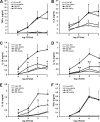
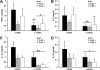
Chitin is a skeletal cell wall polysaccharide of the inner cell wall of fungal pathogens. As yet, little about its role during fungus-host immune cell interactions is known. We show here that ultrapurified chitin from Candida albicans cell walls did not stimulate cytokine production directly but blocked the recognition of C. albicans by human peripheral blood mononuclear cells (PBMCs) and murine macrophages, leading to significant reductions in cytokine production. Chitin did not affect the induction of cytokines stimulated by bacterial cells or lipopolysaccharide (LPS), indicating that blocking was not due to steric masking of specific receptors. Toll-like receptor 2 (TLR2), TLR4, and Mincle (the macrophage-inducible C-type lectin) were not required for interactions with chitin. Dectin-1 was required for immune blocking but did not bind chitin directly. Cytokine stimulation was significantly reduced upon stimulation of PBMCs with heat-killed chitin-deficient C. albicans cells but not with live cells. Therefore, chitin is normally not exposed to cells of the innate immune system but is capable of influencing immune recognition by blocking dectin-1-mediated engagement with fungal cell walls.
Figures



References
-
- Brown G. D., Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

