ATP-dependent and independent functions of Rad54 in genome maintenance
- PMID: 21357745
- PMCID: PMC3051825
- DOI: 10.1083/jcb.201011025
ATP-dependent and independent functions of Rad54 in genome maintenance
Abstract
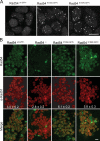
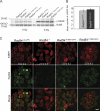
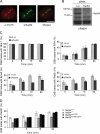
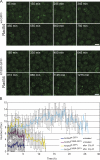
Rad54, a member of the SWI/SNF protein family of DNA-dependent ATPases, repairs DNA double-strand breaks (DSBs) through homologous recombination. Here we demonstrate that Rad54 is required for the timely accumulation of the homologous recombination proteins Rad51 and Brca2 at DSBs. Because replication protein A and Nbs1 accumulation is not affected by Rad54 depletion, Rad54 is downstream of DSB resection. Rad54-mediated Rad51 accumulation does not require Rad54's ATPase activity. Thus, our experiments demonstrate that SWI/SNF proteins may have functions independent of their ATPase activity. However, quantitative real-time analysis of Rad54 focus formation indicates that Rad54's ATPase activity is required for the disassociation of Rad54 from DNA and Rad54 turnover at DSBs. Although the non-DNA-bound fraction of Rad54 reversibly interacts with a focus, independent of its ATPase status, the DNA-bound fraction is immobilized in the absence of ATP hydrolysis by Rad54. Finally, we show that ATP hydrolysis by Rad54 is required for the redistribution of DSB repair sites within the nucleus.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

