Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401
- PMID: 21357782
- PMCID: PMC3083997
- DOI: 10.1200/JCO.2010.30.6985
Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401
Abstract
Purpose: Factors captured in a geriatric assessment can predict morbidity and mortality in older adults, but are not routinely measured in cancer clinical trials. This study evaluated the implementation of a geriatric assessment tool in the cooperative group setting.
Patients and methods: Patients age ≥ 65 with cancer, who enrolled on cooperative group cancer trials, were eligible to enroll on Cancer and Leukemia Group B (CALGB) 360401. They completed a geriatric assessment tool before initiation of protocol therapy, consisting of valid and reliable geriatric assessment measures which are primarily self-administered and require minimal resources and time by healthcare providers. The assessment measures functional status, comorbidity, cognitive function, psychological state, social support, and nutritional status. The protocol specified criteria for incorporation of the tool in future cooperative group trials was based on the time to completion and percent of patients who could complete their portion without assistance. Patient satisfaction with the tool was captured.
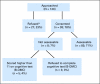
Results: Of the 93 patients who enrolled in this study, five (5%) met criteria for cognitive impairment and three did not complete the cognitive screen, leaving 85 assessable patients (median age, 72 years). The median time to complete the geriatric assessment tool was 22 minutes, 87% of patients (n = 74) completed their portion without assistance, 92% (n = 78) were satisfied with the questionnaire length, 95% (n = 81) reported no difficult questions, and 96% (n = 82) reported no upsetting questions. One hundred percent of health care professionals completed their portion.
Conclusion: This brief, primarily self-administered geriatric assessment tool met the protocol specified criteria for inclusion in future cooperative group clinical trials.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. - PubMed
-
- Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. - PubMed
-
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. - PubMed
-
- Yancik R, Ries LA. Aging and cancer in America: Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. - PubMed
Publication types
MeSH terms
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- CA41287/CA/NCI NIH HHS/United States
- CA45389/CA/NCI NIH HHS/United States
- CA86726/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- K23 AG026749/AG/NIA NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- CA77406/CA/NCI NIH HHS/United States
- CA47577/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- U10 CA086726/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA77651/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA045389/CA/NCI NIH HHS/United States
- CA59518/CA/NCI NIH HHS/United States
- K23 AG026749-01/AG/NIA NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- U10 CA035421/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

