High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases
- PMID: 21358440
- PMCID: PMC3096466
- DOI: 10.1097/MD.0b013e318210e685
High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases
Abstract
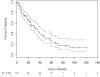
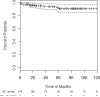
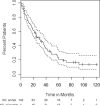
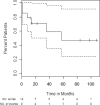
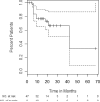
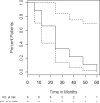
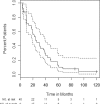
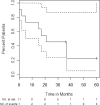
High-dose cyclophosphamide has long been used as an anticancer agent, a conditioning regimen for hematopoietic stem cell transplantation, and a potent immunosuppressive agent in autoimmune diseases including aplastic anemia. High-dose cyclophosphamide is highly toxic to lymphocytes but spares hematopoietic stem cells because of their abundant levels of aldehyde dehydrogenase, the major mechanism of cyclophosphamide inactivation. High-dose cyclophosphamide therapy induces durable remissions in most patients with acquired aplastic anemia. Moreover, high-dose cyclophosphamide without hematopoietic stem cell rescue has shown activity in a variety of other severe autoimmune diseases. Here we review the history of cyclophosphamide as it applies to aplastic anemia and other autoimmune diseases. We include historical data from early patients treated for aplastic anemia as well as data from 140 patients from an observational retrospective study in a single tertiary care hospital. This latter component was designed to assess the safety and efficacy of high-dose cyclophosphamide therapy without stem cell rescue in patients with refractory autoimmune diseases. We analyzed the 140 patients with severe, progressive autoimmune diseases treated. All patients discussed here received cyclophosphamide, 50 mg/kg per day for 4 consecutive days. Response, relapse, and overall survival were measured. Response was defined as a decrease in disease activity in conjunction with a decrease or elimination of immune-modulating drugs. Relapse was defined as worsening disease activity and/or a requirement for an increase in dose of, or administration of new, immunosuppressive medications. Hematologic recovery occurred in all patients. The overall response rate was 94%, and 44% of those patients remained progression free with a median follow-up of 36 months (range, 1-120 mo) for the 140 patients analyzed together. The overall actuarial and event-free survival across all diseases at 60 months was 90.7% and 20.6%, respectively. High-dose cyclophosphamide without stem cell rescue is well tolerated and induces a high rate of remission in severe autoimmune diseases.
Figures

References
-
- Audino AN, Blatt J, Carcamo B, Castaneda V, Dinndorf P, Wang WC, Whitlock JA, Hord JD. High-dose cyclophosphamide treatment for refractory severe aplastic anemia in children. Pediatr Blood Cancer. 2010;54:269–72. - PubMed
-
- Baran DT, Griner PF, Klemperer MR. Recovery from aplastic anemia after treatment with cyclophosphamide. N Engl J Med. 1976;295:1522–3. - PubMed
-
- Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. - PubMed
-
- Brannagan TH, III, Pradhan A, Heiman-Patterson T, Winkelman AC, Styler MJ, Topolsky DL, Crilley PA, Schwartzman RJ, Brodsky I, Gladstone DE. High-dose cyclophosphamide without stem-cell rescue for refractory CIDP. Neurology. 2002;58:1856–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

