Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice
- PMID: 21358676
- PMCID: PMC3141110
- DOI: 10.1038/onc.2011.41
Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice
Abstract
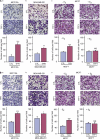
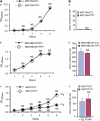
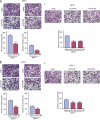
Although trefoil factor 1 (TFF1; previously named pS2) is abnormally expressed in about 50% of human breast tumors, its physiopathological role in this disease has been poorly studied. Moreover, controversial data have been reported. TFF1 function in the mammary gland therefore needs to be clarified. In this study, using retroviral vectors, we performed TFF1 gain- or loss-of-function experiments in four human mammary epithelial cell lines: normal immortalized TFF1-negative MCF10A, malignant TFF1-negative MDA-MB-231 and malignant TFF1-positive MCF7 and ZR75.1. The expression of TFF1 stimulated the migration and invasion in the four cell lines. Forced TFF1 expression in MCF10A, MDA-MB-231 and MCF7 cells did not modify anchorage-dependent or -independent cell proliferation. By contrast, TFF1 knockdown in MCF7 enhanced soft-agar colony formation. This increased oncogenic potential of MCF7 cells in the absence of TFF1 was confirmed in vivo in nude mice. Moreover, chemically induced tumorigenesis in TFF1-deficient (TFF1-KO) mice led to higher tumor incidence in the mammary gland and larger tumor size compared with wild-type mice. Similarly, tumor development was increased in the TFF1-KO ovary and lung. Collectively, our results clearly show that TFF1 does not exhibit oncogenic properties, but rather reduces tumor development. This beneficial function of TFF1 is in agreement with many clinical studies reporting a better outcome for patients with TFF1-positive breast primary tumors.
Figures




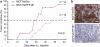
References
-
- Amiry N, Kong X, Muniraj N, Kannan N, Grandison PM, Lin J, et al. Trefoil factor-1 (TFF1) enhances oncogenicity of mammary carcinoma cells. Endocrinology. 2009;150:4473–4483. - PubMed
-
- Buron N, Guery L, Creuzot-Garcher C, Lafontaine PO, Bron A, Rio MC, et al. Trefoil factor TFF1-induced protection of conjunctival cells from apoptosis at premitochondrial and postmitochondrial levels. Invest Ophthalmol Vis Sci. 2008;49:3790–3798. - PubMed
-
- Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing, and glycosylation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904–3909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

