Melatonin metabolism in the central nervous system
- PMID: 21358968
- PMCID: PMC3001211
- DOI: 10.2174/157015910792246244
Melatonin metabolism in the central nervous system
Abstract
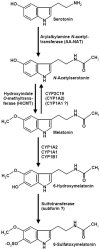
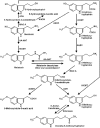
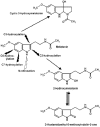
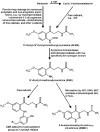
The metabolism of melatonin in the central nervous system is of interest for several reasons. Melatonin enters the brain either via the pineal recess or by uptake from the blood. It has been assumed to be also formed in some brain areas. Neuroprotection by melatonin has been demonstrated in numerous model systems, and various attempts have been undertaken to counteract neurodegeneration by melatonin treatment. Several concurrent pathways lead to different products. Cytochrome P(450) subforms have been demonstrated in the brain. They either demethylate melatonin to N-acetylserotonin, or produce 6-hydroxymelatonin, which is mostly sulfated already in the CNS. Melatonin is deacetylated, at least in pineal gland and retina, to 5-methoxytryptamine. N(1)-acetyl-N(2)-formyl-5-methoxykynuramine is formed by pyrrole-ring cleavage, by myeloperoxidase, indoleamine 2,3-dioxygenase and various non-enzymatic oxidants. Its product, N(1)-acetyl-5-methoxykynuramine, is of interest as a scavenger of reactive oxygen and nitrogen species, mitochondrial modulator, downregulator of cyclooxygenase-2, inhibitor of cyclooxygenase, neuronal and inducible NO synthases. Contrary to other nitrosated aromates, the nitrosated kynuramine metabolite, 3-acetamidomethyl-6-methoxycinnolinone, does not re-donate NO. Various other products are formed from melatonin and its metabolites by interaction with reactive oxygen and nitrogen species. The relative contribution of the various pathways to melatonin catabolism seems to be influenced by microglia activation, oxidative stress and brain levels of melatonin, which may be strongly changed in experiments on neuroprotection. Many of the melatonin metabolites, which may appear in elevated concentrations after melatonin administration, possess biological or pharmacological properties, including N-acetylserotonin, 5-methoxytryptamine and some of its derivatives, and especially the 5-methoxylated kynuramines.
Keywords: 5-methoxytryptamine; 6-sulfatoxymelatonin.; Kynuramines; N-acetylserotonin; melatonin; reactive nitrogen species; reactive oxygen species.
Figures
References
-
- Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H. Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 2003;527:549–557. - PubMed
-
- Agozzino P, Avellone G, Bongiorno D, Ceraulo L, Filizzola F, Natoli MC, Livrea MA, Tesoriere L. Melatonin: structural characterization of its non-enzymatic mono-oxygenate metabolite. J. Pineal Res. 2003;35:269–275. - PubMed
-
- Agrawal R, Tyagi E, Shukla R, Nath C. Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav. Brain Res. 2008;189:381–386. - PubMed
-
- Akimov MG, Nazimov IV, Gretskaya NM, Zinchenko GN, Bezuglov VV. Sulfation of N-acyl dopamines in rat tissues. Biochemistry (Mosc.) 2009;74:681–685. - PubMed
-
- Backhaus C, Rahman H, Scheffler S, Laatsch H, Hardeland R. NO scavenging by 3-hydroxyanthranilic acid and 3-hydroxykynurenine: N-nitrosation leads via oxadiazoles to o-quinone diazides. Nitric Oxide. 2008;19:237–244. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
