Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism
- PMID: 21359227
- PMCID: PMC3040206
- DOI: 10.1371/journal.pone.0017116
Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism
Abstract
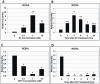
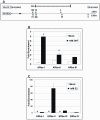
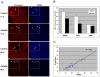
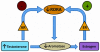
Autism, a pervasive neurodevelopmental disorder manifested by deficits in social behavior and interpersonal communication, and by stereotyped, repetitive behaviors, is inexplicably biased towards males by a ratio of ∼4∶1, with no clear understanding of whether or how the sex hormones may play a role in autism susceptibility. Here, we show that male and female hormones differentially regulate the expression of a novel autism candidate gene, retinoic acid-related orphan receptor-alpha (RORA) in a neuronal cell line, SH-SY5Y. In addition, we demonstrate that RORA transcriptionally regulates aromatase, an enzyme that converts testosterone to estrogen. We further show that aromatase protein is significantly reduced in the frontal cortex of autistic subjects relative to sex- and age-matched controls, and is strongly correlated with RORA protein levels in the brain. These results indicate that RORA has the potential to be under both negative and positive feedback regulation by male and female hormones, respectively, through one of its transcriptional targets, aromatase, and further suggest a mechanism for introducing sex bias in autism.
Conflict of interest statement
Figures

References
-
- American Psychological Association. Washington, DC: American Psychological Association; 1994. Diagnostic and statistical manual of mental disorders.
-
- Volkmar FR, Klin A, Siegel B, Szatmari P, Lord C, et al. Field trial for autistic disorder in DSM-IV. Am J Psychiatry. 1994;151(9):1361–7. - PubMed
-
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: Implications for explaining autism. Science. 2005;310(5749):819–23. - PubMed
-
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, et al. Fetal testosterone and autistic traits. Br J Psychol. 2009;100(1):1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

