Genomics of the NF-κB signaling pathway: hypothesized role in ovarian cancer
- PMID: 21359843
- PMCID: PMC3119514
- DOI: 10.1007/s10552-011-9745-4
Genomics of the NF-κB signaling pathway: hypothesized role in ovarian cancer
Abstract
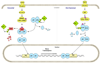
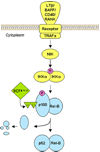
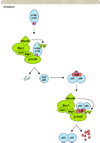
Objective: We sought to review evidence linking nuclear factor-kappa B (NF-κB) to ovarian cancer and to identify genetic variants involved in NF-κB signaling.
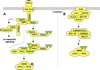
Methods: PubMed was reviewed to inform on ovarian cancer biology and NF-κB signaling and to identify key genes. Public linkage disequilibrium (LD) data were analyzed to identify informative inherited variants (tagSNPs) using ldSelect.
Results: We identified 319 key NF-κB genes including five NF-κB subunits, 167 activating genes, and 55 inhibiting genes. We found that the 1000 Genomes Project was the most informative LD source for most genes (92.8%), and we identified 13,027 LD bins (r (2) ≥ 0.9, minor allele frequency ≥ 0.05) and 1,018 putative-functional variants worthy of investigation. We also report that reliance on a commonly used genome-wide SNP array and genotype imputation with HapMap Phase II data provides data on only 74% of the common inherited NF-κB SNPs of interest.
Conclusions: Compelling evidence suggests that NF-κB plays a critical role in ovarian cancer, yet inherited variation in these genes has not been thoroughly assessed in relation to disease risk or outcome. We present a collection of variants in key genes and suggest creation of a custom genotyping array as an optimal approach.
Figures


Similar articles
-
Activation of NF-kappaB signaling by inhibitor of NF-kappaB kinase beta increases aggressiveness of ovarian cancer.Cancer Res. 2010 May 15;70(10):4005-14. doi: 10.1158/0008-5472.CAN-09-3912. Epub 2010 Apr 27. Cancer Res. 2010. PMID: 20424119 Free PMC article.
-
Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer.Cancer. 2010 Jul 1;116(13):3276-84. doi: 10.1002/cncr.25190. Cancer. 2010. PMID: 20564628 Free PMC article.
-
The NF-κB pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC).Gynecol Oncol. 2011 Oct;123(1):129-37. doi: 10.1016/j.ygyno.2011.06.006. Epub 2011 Jul 22. Gynecol Oncol. 2011. PMID: 21782227
-
The nuclear factor-kappa B pathway and response to treatment in breast cancer.Pharmacogenomics. 2017 Dec;18(18):1697-1709. doi: 10.2217/pgs-2017-0044. Epub 2017 Nov 28. Pharmacogenomics. 2017. PMID: 29182047 Review.
-
Classic and Novel Signaling Pathways Involved in Cancer: Targeting the NF-κB and Syk Signaling Pathways.Curr Stem Cell Res Ther. 2019;14(3):219-225. doi: 10.2174/1574888X13666180723104340. Curr Stem Cell Res Ther. 2019. PMID: 30033874 Review.
Cited by
-
Symbiotic prodrugs (SymProDs) dual targeting of NFkappaB and CDK.Chem Biol Drug Des. 2020 Aug;96(2):773-784. doi: 10.1111/cbdd.13684. Epub 2020 Apr 22. Chem Biol Drug Des. 2020. PMID: 32237047 Free PMC article.
-
Variation in NF-κB signaling pathways and survival in invasive epithelial ovarian cancer.Cancer Epidemiol Biomarkers Prev. 2014 Jul;23(7):1421-7. doi: 10.1158/1055-9965.EPI-13-0962. Epub 2014 Apr 16. Cancer Epidemiol Biomarkers Prev. 2014. PMID: 24740199 Free PMC article.
-
The Unique Molecular and Cellular Microenvironment of Ovarian Cancer.Front Oncol. 2017 Feb 22;7:24. doi: 10.3389/fonc.2017.00024. eCollection 2017. Front Oncol. 2017. PMID: 28275576 Free PMC article. Review.
-
A High-Fat and High-Carbohydrate Diet Promotes Reminiscent Hallmarks of an Aging Ovary in the Rabbit Model.Biomedicines. 2022 Nov 29;10(12):3068. doi: 10.3390/biomedicines10123068. Biomedicines. 2022. PMID: 36551824 Free PMC article.
-
Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial.Gynecol Oncol. 2014 Nov;135(2):297-304. doi: 10.1016/j.ygyno.2014.08.025. Epub 2014 Aug 23. Gynecol Oncol. 2014. PMID: 25158036 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. - PubMed
-
- Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47(2):159–166. - PubMed
-
- McGuire V, Jesser CA, Whittemore AS. Survival among U.S. women with invasive epithelial ovarian cancer. Gynecol Oncol. 2002;84(3):399–403. - PubMed
-
- Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. 2003;189(4):1120–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

