Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury
- PMID: 21360570
- PMCID: PMC3082595
- DOI: 10.1002/hep.24253
Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury
Abstract
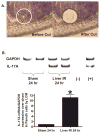
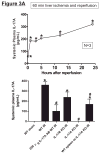
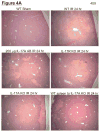
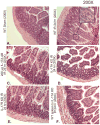
Hepatic ischemia and reperfusion (IR) injury is a major clinical problem that leads to frequent extrahepatic complications including intestinal dysfunction and acute kidney injury (AKI). In this study we aimed to determine the mechanisms of hepatic IR-induced extrahepatic organ dysfunction. Mice subjected to 60 minutes of hepatic IR not only developed severe hepatic injury but also developed significant AKI and small intestinal injury. Hepatic IR induced small intestinal Paneth cell degranulation and increased interleukin-17A (IL-17A) levels in portal vein plasma and small intestine. We also detected increased levels of IL-17A messenger RNA (mRNA) and protein in Paneth cells after hepatic IR with laser capture dissection. IL-17A-neutralizing antibody treatment or genetic deletion of either IL-17A or IL-17A receptors significantly protected against hepatic IR-induced acute liver, kidney, and intestinal injury. Leukocyte IL-17A does not contribute to organ injury, as infusion of wildtype splenocytes failed to exacerbate liver and kidney injury in IL-17A-deficient mice after hepatic IR. Depletion of Paneth cell numbers by pharmacological (with dithizone) or genetic intervention (SOX9 flox/flox Villin cre+/- mice) significantly attenuated intestinal, hepatic, and renal injury following liver IR. Finally, depletion of Paneth cell numbers significantly decreased small intestinal IL-17A release and plasma IL-17A levels after liver IR.
Conclusion: Taken together, the results show that Paneth cell-derived IL-17A plays a critical role in hepatic IR injury and extrahepatic organ dysfunction. Modulation of Paneth cell dysregulation may have therapeutic implications by reducing systemic complications arising from hepatic IR.
Copyright © 2011 American Association for the Study of Liver Diseases.
Conflict of interest statement
None of the authors had financial interests or ties to commercial companies.
Figures















References
-
- Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005 May;37(4):1653–1656. - PubMed
-
- Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part I. Liver Transpl. 2002 Feb;8(2):91–109. - PubMed
-
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010 Jul;10(7):479–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
