Structure and reaction mechanism in the heme dioxygenases
- PMID: 21361337
- PMCID: PMC3092302
- DOI: 10.1021/bi101732n
Structure and reaction mechanism in the heme dioxygenases
Abstract
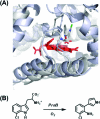
As members of the family of heme-dependent enzymes, the heme dioxygenases are differentiated by virtue of their ability to catalyze the oxidation of l-tryptophan to N-formylkynurenine, the first and rate-limiting step in tryptophan catabolism. In the past several years, there have been a number of important developments that have meant that established proposals for the reaction mechanism in the heme dioxygenases have required reassessment. This focused review presents a summary of these recent advances, written from a structural and mechanistic perspective. It attempts to present answers to some of the long-standing questions, to highlight as yet unresolved issues, and to explore the similarities and differences of other well-known catalytic heme enzymes such as the cytochromes P450, NO synthase, and peroxidases.
Figures




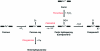
References
-
- Kotake Y.; Masayama I. (1936) The intermediary metabolism of tryptophan. XVIII. The mechanism of formation of kynurenine from tryptophan. Z. Physiol. Chem. 243, 237–244.
-
- Yamamoto S.; Hayaishi O. (1967) Tryptophan pyrrolase of rabbit intestine. d- and l-tryptophan-cleaving enzyme or enzymes. J. Biol. Chem. 242, 5260–5266. - PubMed
-
- Tanaka T.; Knox W. E. (1959) The nature and mechanism of the tryptophan pyrrolase (peroxidase-oxidase) reaction of Pseudomonas and of rat liver. J. Biol. Chem. 234, 1162–1170. - PubMed
-
- Knox W. E.; Mehler A. H. (1950) The conversion of tryptophan to kynurenine in liver. I. The coupled tryptophan peroxidase-oxidase system forming formylkynurenine. J. Biol. Chem. 187, 419–430. - PubMed
-
- Sono M.; Roach M. P.; Coulter E. D.; Dawson J. H. (1996) Heme-Containing Oxygenases. Chem. Rev. 96, 2841–2887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

