Maximum imaging depth of two-photon autofluorescence microscopy in epithelial tissues
- PMID: 21361692
- PMCID: PMC3061332
- DOI: 10.1117/1.3548646
Maximum imaging depth of two-photon autofluorescence microscopy in epithelial tissues
Abstract
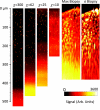
Endogenous fluorescence provides morphological, spectral, and lifetime contrast that can indicate disease states in tissues. Previous studies have demonstrated that two-photon autofluorescence microscopy (2PAM) can be used for noninvasive, three-dimensional imaging of epithelial tissues down to approximately 150 μm beneath the skin surface. We report ex-vivo 2PAM images of epithelial tissue from a human tongue biopsy down to 370 μm below the surface. At greater than 320 μm deep, the fluorescence generated outside the focal volume degrades the image contrast to below one. We demonstrate that these imaging depths can be reached with 160 mW of laser power (2-nJ per pulse) from a conventional 80-MHz repetition rate ultrafast laser oscillator. To better understand the maximum imaging depths that we can achieve in epithelial tissues, we studied image contrast as a function of depth in tissue phantoms with a range of relevant optical properties. The phantom data agree well with the estimated contrast decays from time-resolved Monte Carlo simulations and show maximum imaging depths similar to that found in human biopsy results. This work demonstrates that the low staining inhomogeneity (∼ 20) and large scattering coefficient (∼ 10 mm(-1)) associated with conventional 2PAM limit the maximum imaging depth to 3 to 5 mean free scattering lengths deep in epithelial tissue.
Figures







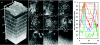

References
-
- Zipfel W. R., Williams R. M., Christie R., Nikitin A. Y., Hyman B. T., and Webb W. W., “Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation,” Proc. Nat. Acad. Sci. U.S.A. 100, 7075–7080 (2003).10.1073/pnas.0832308100 - DOI - PMC - PubMed
-
- Skala M. C., Squirrell J. M., Vrotsos K. M., Eickhoff J. C., Gendron-Fitzpatrick A., Eliceiri K. W., and Ramanujam N., “Multiphoton microscopy of endogenous fluorescence differentiates normal, precancerous, and cancerous squamous epithelial tissues,” Cancer Res. 65, 1180–1186 (2005).10.1158/0008-5472.CAN-04-3031 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

