3-D photoacoustic and pulse echo imaging of prostate tumor progression in the mouse window chamber
- PMID: 21361696
- PMCID: PMC3065344
- DOI: 10.1117/1.3540668
3-D photoacoustic and pulse echo imaging of prostate tumor progression in the mouse window chamber
Abstract
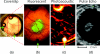
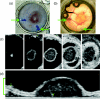
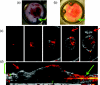
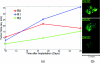
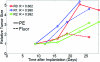
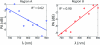
Understanding the tumor microenvironment is critical to characterizing how cancers operate and predicting their response to treatment. We describe a novel, high-resolution coregistered photoacoustic (PA) and pulse echo (PE) ultrasound system used to image the tumor microenvironment. Compared to traditional optical systems, the platform provides complementary contrast and important depth information. Three mice are implanted with a dorsal skin flap window chamber and injected with PC-3 prostate tumor cells transfected with green fluorescent protein. The ensuing tumor invasion is mapped during three weeks or more using simultaneous PA and PE imaging at 25 MHz, combined with optical and fluorescent techniques. Pulse echo imaging provides details of tumor structure and the surrounding environment with 100-μm(3) resolution. Tumor size increases dramatically with an average volumetric growth rate of 5.35 mm(3)/day, correlating well with 2-D fluorescent imaging (R = 0.97, p < 0.01). Photoacoustic imaging is able to track the underlying vascular network and identify hemorrhaging, while PA spectroscopy helps classify blood vessels according to their optical absorption spectrum, suggesting variation in blood oxygen saturation. Photoacoustic and PE imaging are safe, translational modalities that provide enhanced depth resolution and complementary contrast to track the tumor microenvironment, evaluate new cancer therapies, and develop molecular contrast agents in vivo.
Figures








References
-
- Kawachi M. H., Bahnson R. R., Barry M., Busby J. E., Carroll P. R., Carter H. B., Catalona W. J., Cookson M. S., Epstein J. I., Etzioni R. B., Giri V. N., Hemstreet G. P., Howe R. J., Lange P. H., Lilja H., Loughlin K. R., Mohler J., Moul J., Nadler R. B., Patterson S. G., Presti J. C., Stroup A. M., Wake R., and Wei J. T., “Prostate cancer early detection,” J. Natl. Comprehens. Cancer Netw. 8(2), 240–262 (2010). http://www.jnccn.org/content/8/2/240.short - PubMed
-
- Bauer D. R., Olafsson R., Montilla L. G. and Witte R. S., “In vivo multi-modality photoacoustic and pulse echo tracking of prostate tumor growth using a window chamber,” Proc. SPIE 7564, 75643B (2010).10.1117/12.843875 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

