Dynamic reciprocity in the wound microenvironment
- PMID: 21362080
- PMCID: PMC3051353
- DOI: 10.1111/j.1524-475X.2011.00673.x
Dynamic reciprocity in the wound microenvironment
Abstract
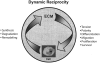
Here, we define dynamic reciprocity (DR) as an ongoing, bidirectional interaction among cells and their surrounding microenvironment. In this review, we posit that DR is especially meaningful during wound healing as the DR-driven biochemical, biophysical, and cellular responses to injury play pivotal roles in regulating tissue regenerative responses. Such cell-extracellular matrix interactions not only guide and regulate cellular morphology, but also cellular differentiation, migration, proliferation, and survival during tissue development, including, e.g., embryogenesis, angiogenesis, as well as during pathologic processes including cancer, diabetes, hypertension, and chronic wound healing. Herein, we examine DR within the wound microenvironment while considering specific examples across acute and chronic wound healing. This review also considers how a number of hypotheses that attempt to explain chronic wound pathophysiology may be understood within the DR framework. The implications of applying the principles of DR to optimize wound care practice and future development of innovative wound healing therapeutics are also briefly considered.
© 2011 by the Wound Healing Society.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- K08 EY019533/EY/NEI NIH HHS/United States
- DK065656/DK/NIDDK NIH HHS/United States
- EY05587/EY/NEI NIH HHS/United States
- EY15125/EY/NEI NIH HHS/United States
- AR056138/AR/NIAMS NIH HHS/United States
- R01 EY005587/EY/NEI NIH HHS/United States
- R01 AR056138/AR/NIAMS NIH HHS/United States
- R01 DK065656/DK/NIDDK NIH HHS/United States
- R21 EY019553/EY/NEI NIH HHS/United States
- R01 EY015125/EY/NEI NIH HHS/United States
- R01 AG006528/AG/NIA NIH HHS/United States
- P30 EY021721/EY/NEI NIH HHS/United States
- EY19533/EY/NEI NIH HHS/United States
- AG06528/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

