Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo
- PMID: 21362406
- PMCID: PMC3123253
- DOI: 10.1016/j.bbrc.2011.02.113
Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo
Abstract
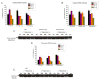
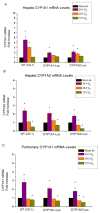
Supplemental oxygen administration is frequently administered to pre-term and term infants having pulmonary insufficiency. However, hyperoxia contributes to the development of bronchopulmonary dysplasia (BPD) in premature infants. Cytochrome P450 (CYP)A enzymes have been implicated in hyperoxic lung injury. In this study, we tested the hypothesis that hyperoxia induces CYP1A1 and 1A2 enzymes by transcriptional activation of the corresponding promoters in vivo, and transgenic mice expressing the human CYP1A1 or the mouse 1A2 promoter would be more susceptible to hyperoxic lung injury than wild type (WT) mice. Adult WT (CD-1) (12week-old) mice, transgenic mice carrying a 10kb human CYP1A1 promoter and the luciferase (luc) reporter gene (CYP1A1-luc), or mice expressing the mouse CYP1A2 promoter (CYP1A2-luc) were maintained in room air or exposed to hyperoxia for 24-72h. Hyperoxia exposure of CYP1A1-luc mice for 24 and 48h resulted in 2.5- and 1.25-fold increases, respectively, in signal intensities, compared to room air controls. By 72h, the induction had declined to control levels. CYP1A2-luc mice also showed enhanced luc expression after 24-48h, albeit to a lesser extent than those expressing the CYP1A1 promoter. Also, these mice showed decreased levels of endogenous CYP1A1 and 1A2 expression after prolonged hyperoxia, and were also more susceptible to lung injury than similarly exposed WT mice, with CYP1A2-luc mice showing the greatest injury. Our results support the hypothesis that hyperoxia induces CYP1A enzymes by transcriptional activation of its corresponding promoters, and that decreased endogenous expression of these enzymes contribute to the increased susceptibilities to hyperoxic lung injury in the transgenic animals. In summary, this is the first report providing direct evidence of hyperoxia-mediated induction of CYP1A1 and CYP1A2 expression in vivo by mechanisms entailing transcriptional activation of the corresponding promoters, a phenomenon that has implications for hyperoxic lung injury, as well as other pathologies caused by oxidative stress.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Northway WH, Jr, Rosan RC. Radiographic features of pulmonary oxygen toxicity in the newborn: Bronchopulmonary dysplasia. Radiology. 1968;91:49–58. - PubMed
-
- Fisher AB. Oxygen therapy. Side effects and toxicity. Am Rev Respir Dis. 1980;122:61–69. - PubMed
-
- Bland RD, Coalson JJ. Chronic lung disease in early infancy. M. Dekker; New York: 2000.
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. - PubMed
-
- Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

