Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death
- PMID: 21362471
- PMCID: PMC3081988
- DOI: 10.1016/j.freeradbiomed.2011.02.021
Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death
Abstract
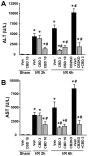
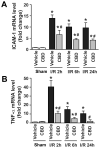
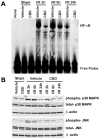
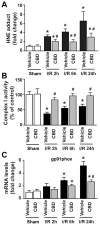
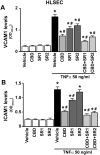
Ischemia/reperfusion (I/R) is a pivotal mechanism of liver damage after liver transplantation or hepatic surgery. We have investigated the effects of cannabidiol (CBD), the nonpsychotropic constituent of marijuana, in a mouse model of hepatic I/R injury. I/R triggered time-dependent increases/changes in markers of liver injury (serum transaminases), hepatic oxidative/nitrative stress (4-hydroxy-2-nonenal, nitrotyrosine content/staining, and gp91phox and inducible nitric oxide synthase mRNA), mitochondrial dysfunction (decreased complex I activity), inflammation (tumor necrosis factor α (TNF-α), cyclooxygenase 2, macrophage inflammatory protein-1α/2, intercellular adhesion molecule 1 mRNA levels; tissue neutrophil infiltration; nuclear factor κB (NF-κB) activation), stress signaling (p38MAPK and JNK), and cell death (DNA fragmentation, PARP activity, and TUNEL). CBD significantly reduced the extent of liver inflammation, oxidative/nitrative stress, and cell death and also attenuated the bacterial endotoxin-triggered NF-κB activation and TNF-α production in isolated Kupffer cells, likewise the adhesion molecule expression in primary human liver sinusoidal endothelial cells stimulated with TNF-α and attachment of human neutrophils to the activated endothelium. These protective effects were preserved in CB2 knockout mice and were not prevented by CB1/2 antagonists in vitro. Thus, CBD may represent a novel, protective strategy against I/R injury by attenuating key inflammatory pathways and oxidative/nitrative tissue injury, independent of classical CB1/2 receptors.
Published by Elsevier Inc.
Conflict of interest statement
Figures









References
-
- Liaudet L, Szabo G, Szabo C. Oxidative stress and regional ischemia-reperfusion injury: the peroxynitrite-poly(ADP-ribose) polymerase connection. Coron Artery Dis. 2003;14:115–122. - PubMed
-
- Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

