The evolution of imaging in cancer: current state and future challenges
- PMID: 21362512
- PMCID: PMC4313866
- DOI: 10.1053/j.seminoncol.2010.11.010
The evolution of imaging in cancer: current state and future challenges
Abstract
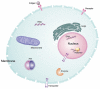
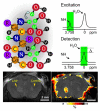
Molecular imaging allows for the remote, noninvasive sensing and measurement of cellular and molecular processes in living subjects. Drawing upon a variety of modalities, molecular imaging provides a window into the biology of cancer from the subcellular level to the patient undergoing a new, experimental therapy. As signal transduction cascades and protein interaction networks become clarified, an increasing number of relevant targets for cancer therapy--and imaging--become available. Although conventional imaging is already critical to the management of patients with cancer, molecular imaging will provide even more relevant information, such as early detection of changes with therapy, identification of patient-specific cellular and metabolic abnormalities, and the disposition of therapeutic, gene-tagged cells throughout the body--all of which will have a considerable impact on morbidity and mortality. This overview discusses molecular imaging in oncology, providing examples from a variety of modalities, with an emphasis on emerging techniques for translational imaging.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5:220–33. - PubMed
-
- Kwee TC, Takahara T, Klomp DW, Luijten PR. Cancer imaging: novel concepts in clinical magnetic resonance imaging. J Intern Med. 2010;268:120–32. - PubMed
-
- Kovacs A, Toth L, Glavak C, et al. Integrating functional MRI information into radiotherapy planning of CNS tumors—early experiences. Pathol Oncol Res. Epub ahead of print 2010 September 17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

