Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells
- PMID: 21362570
- PMCID: PMC3061351
- DOI: 10.1016/j.stem.2011.01.012
Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells
Abstract
Embryonic stem cells (ESCs) are an attractive source for tissue regeneration and repair therapies because they can be differentiated into virtually any cell type in the adult body. However, for this approach to succeed, the transplanted ESCs must survive long enough to generate a therapeutic benefit. A major obstacle facing the engraftment of ESCs is transplant rejection by the immune system. Here we show that blocking leukocyte costimulatory molecules permits ESC engraftment. We demonstrate the success of this immunosuppressive therapy for mouse ESCs, human ESCs, mouse induced pluripotent stem cells (iPSCs), human induced pluripotent stem cells, and more differentiated ESC/(iPSCs) derivatives. Additionally, we provide evidence describing the mechanism by which inhibition of costimulatory molecules suppresses T cell activation. This report describes a short-term immunosuppressive approach capable of inducing engraftment of transplanted ESCs and iPSCs, providing a significant improvement in our mechanistic understanding of the critical role costimulatory molecules play in leukocyte activation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
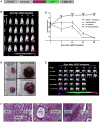


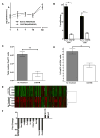
Comment in
-
Safely modulating the immune system in regenerative medicine.Cell Stem Cell. 2011 Mar 4;8(3):246-7. doi: 10.1016/j.stem.2011.02.013. Cell Stem Cell. 2011. PMID: 21362563
References
-
- Abdullah Z, Saric T, Kashkar H, Baschuk N, Yazdanpanah B, Fleischmann BK, Hescheler J, Kronke M, Utermohlen O. Serpin-6 expression protects embryonic stem cells from lysis by antigen-specific CTL. J Immunol. 2007;178:3390–3399. - PubMed
-
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. - PubMed
-
- Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24:2192–2201. - PubMed
-
- Byrne JA. Generation of isogenic pluripotent stem cells. Hum Mol Genet. 2008;17:R37–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

