Isolation of epiblast stem cells from preimplantation mouse embryos
- PMID: 21362571
- PMCID: PMC3073125
- DOI: 10.1016/j.stem.2011.01.016
Isolation of epiblast stem cells from preimplantation mouse embryos
Abstract
Pluripotent stem cells provide a platform to interrogate control elements that function to generate all cell types of the body. Despite their utility for modeling development and disease, the relationship of mouse and human pluripotent stem cell states to one another remains largely undefined. We have shown that mouse embryonic stem (ES) cells and epiblast stem cells (EpiSCs) are distinct, pluripotent states isolated from pre- and post-implantation embryos respectively. Human ES cells are different than mouse ES cells and share defining features with EpiSCs, yet are derived from pre-implantation human embryos. Here we show that EpiSCs can be routinely derived from pre-implantation mouse embryos. The preimplantation-derived EpiSCs exhibit molecular features and functional properties consistent with bona fide EpiSCs. These results provide a simple method for isolating EpiSCs and offer direct insight into the intrinsic and extrinsic mechanisms that regulate the acquisition of distinct pluripotent states.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
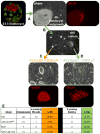

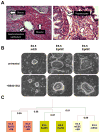
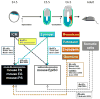
Comment in
-
Primed for pluripotency.Cell Stem Cell. 2011 Mar 4;8(3):241-2. doi: 10.1016/j.stem.2011.02.009. Cell Stem Cell. 2011. PMID: 21362560
References
-
- Aoki H, Hara A, Niwa M, Yamada Y, Kunisada T. In vitro and in vivo differentiation of human embryonic stem cells into retina-like organs and comparison with that from mouse pluripotent epiblast stem cells. Dev Dyn. 2009;238:2266–2279. - PubMed
-
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
-
- Brook FA, Evans EP, Lord CJ, Lyons PA, Rainbow DB, Howlett SK, Wicker LS, Todd JA, Gardner RL. The Derivation of Highly Germline-Competent Embryonic Stem Cells Containing NOD-Derived Genome. Diabetes. 2003;52:205–208. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

