Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer
- PMID: 21363919
- PMCID: PMC3076144
- DOI: 10.1158/0008-5472.CAN-10-3166
Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer
Abstract
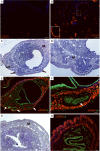
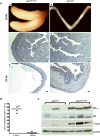
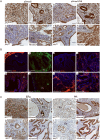
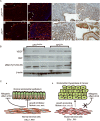
The contribution of the stromal microenvironment to the progression of endometrial cancer has not been well explored. We have conditionally expressed a mutant allele of adenomatous polyposis coli (APC(cKO)) in murine uterine stroma cells to study its effect on uterine development and function. In addition to metrorrhagia, the mice develop complex atypical endometrial gland hyperplasia that progresses to endometrial carcinoma in situ and endometrial adenocarcinoma as evidenced by myometrial invasion. Stromal cells subjacent to the carcinoma cells express alpha-smooth muscle actin (αSMA) with fewer cells expressing platelet-derived growth factor α compared with normal stromal cells, suggesting that the mutant stromal cells have acquired a more myofibroblastic phenotype, which have been described as cancer-associated fibroblasts and have been shown to induce carcinogenesis in other organ systems. Analyses of human endometrial cancer specimens showed substantial αSMA expression in the stroma compared with normal endometrial stroma cells. We also show that APC(cKO) mutant uteri and human endometrial cancer have decreased stromal levels of transforming growth factor β and bone morphogenetic protein activities and that the mutant uteri failed to respond to exogenous estradiol stimulation. The mutant stroma cells also had higher levels of vascular endothelial growth factor and stromal derived factor signaling components and diminished expression of estrogen receptor α and progesterone receptor, which is common in advanced stages of human endometrial cancer and is an indicator of poor prognosis. Our results indicate that de novo mutation or loss of heterozygosity in stromal APC is sufficient to induce endometrial hyperplasia and endometrial carcinogenesis by mechanisms that are consistent with unopposed estrogen signaling in the endometrial epithelium.
©2011 AACR.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

