Distinct TRAIL resistance mechanisms can be overcome by proteasome inhibition but not generally by synergizing agents
- PMID: 21363923
- PMCID: PMC3250348
- DOI: 10.1158/0008-5472.CAN-10-2252
Distinct TRAIL resistance mechanisms can be overcome by proteasome inhibition but not generally by synergizing agents
Abstract
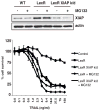
One impediment to the use of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-targeted agents as antitumor drugs is the evolution of resistance, a common problem in cancer. On the contrary, many different kinds of drugs synergize with TRAIL in TRAIL-sensitive tumor cells, raising the question whether one can overcome resistance with the same drugs producing synergy. This is an important question, because recent clinical trials suggest that combination treatments with cytotoxic drugs and TRAIL receptor-targeted agents do not provide additional benefit compared with cytotoxic agents on their own. Such results might be expected if drug combinations that synergize in sensitive tumor cells but cannot overcome TRAIL resistance are used in patients whose tumors were not selected for retention of TRAIL sensitivity. We tested this idea by creating isogenic tumor cells with acquired TRAIL resistance or defined mechanisms of resistance that occur in human tumors and then comparing them to the TRAIL-sensitive parental cell line. Although diverse classes of anticancer drugs were all able to synergize with TRAIL in sensitive cells, most agents were unable to overcome resistance and there was no relationship between the amount of synergy seen with a particular agent and its ability to overcome acquired resistance. An important exception was proteasome inhibitors, which were, however, able to overcome diverse resistance mechanisms. Our findings suggest that one should select drugs for TRAIL receptor agonist combination therapy based not just on their ability to synergize, but rather on their ability to overcome resistance as well as synergize.
©2011 AACR.
Figures




References
-
- Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. - PubMed
-
- Thorburn A. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway signaling. J Thorac Oncol. 2007;2:461–5. - PubMed
-
- Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. - PubMed
-
- Koschny R, Holland H, Sykora J, et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13:3403–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

