Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult Mammalian brain
- PMID: 21363934
- PMCID: PMC3179409
- DOI: 10.1210/en.2010-1396
Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult Mammalian brain
Abstract
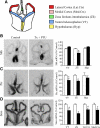
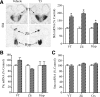
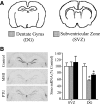
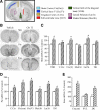
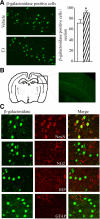
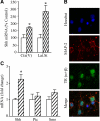
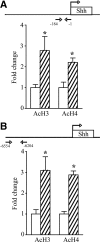
Thyroid hormone is important for development and plasticity in the immature and adult mammalian brain. Several thyroid hormone-responsive genes are regulated during specific developmental time windows, with relatively few influenced across the lifespan. We provide novel evidence that thyroid hormone regulates expression of the key developmental morphogen sonic hedgehog (Shh), and its coreceptors patched (Ptc) and smoothened (Smo), in the early embryonic and adult forebrain. Maternal hypo- and hyperthyroidism bidirectionally influenced Shh mRNA in embryonic forebrain signaling centers at stages before fetal thyroid hormone synthesis. Further, Smo and Ptc expression were significantly decreased in the forebrain of embryos derived from hypothyroid dams. Adult-onset thyroid hormone perturbations also regulated expression of the Shh pathway bidirectionally, with a significant induction of Shh, Ptc, and Smo after hyperthyroidism and a decline in Smo expression in the hypothyroid brain. Short-term T₃ administration resulted in a significant induction of cortical Shh mRNA expression and also enhanced reporter gene expression in Shh(+/LacZ) mice. Further, acute T₃ treatment of cortical neuronal cultures resulted in a rapid and significant increase in Shh mRNA, suggesting direct effects. Chromatin immunoprecipitation assays performed on adult neocortex indicated enhanced histone acetylation at the Shh promoter after acute T₃ administration, providing further support that Shh is a thyroid hormone-responsive gene. Our results indicate that maternal and adult-onset perturbations of euthyroid status cause robust and region-specific changes in the Shh pathway in the embryonic and adult forebrain, implicating Shh as a possible mechanistic link for specific neurodevelopmental effects of thyroid hormone.
Figures
References
-
- Porterfield SP, Hendrich CE. 1993. The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev 14:94–106 - PubMed
-
- Anderson GW, Schoonover CM, Jones SA. 2003. Control of thyroid hormone action in the developing rat brain. Thyroid 13:1039–1056 - PubMed
-
- Anderson GW. 2001. Thyroid hormones and the brain. Front Neuroendocrinol 22:1–17 - PubMed
-
- Akaike M, Kato N, Ohno H, Kobayashi T. 1991. Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol 13:317–322 - PubMed
-
- Williams GR. 2008. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

