Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial
- PMID: 21364138
- PMCID: PMC3403835
- DOI: 10.1001/jama.2011.235
Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial
Abstract
Context: Inhaled nitric oxide has shown evidence of efficacy in mouse models of sickle cell disease (SCD), case series of patients with acute chest syndrome, and 2 small placebo-controlled trials for treatment of vaso-occlusive pain crisis (VOC).
Objective: To determine whether inhaled nitric oxide gas reduces the duration of painful crisis in patients with SCD who present to the emergency department or hospital for care.
Design, setting, and participants: Prospective, multicenter, double-blind, randomized, placebo-controlled clinical trial for up to 72 hours of inhaled nitric oxide gas vs inhaled nitrogen placebo in 150 participants presenting with VOC of SCD at 11 centers between October 5, 2004, and December 22, 2008. Intervention Inhaled nitric oxide gas vs inhaled nitrogen placebo.
Main outcome measures: The primary end point was the time to resolution of painful crisis, defined by (1) freedom from parenteral opioid use for 5 hours; (2) pain relief as assessed by visual analog pain scale scores of 6 cm or lower (on 0-10 scale); (3) ability to walk; and (4) patient's and family's decision, with physician consensus, that the remaining pain could be managed at home.
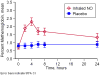
Results: There was no significant change in the primary end point between the nitric oxide and placebo groups, with a median time to resolution of crisis of 73.0 hours (95% confidence interval [CI], 46.0-91.0) and 65.5 hours (95% CI, 48.1-84.0), respectively (P = .87). There were no significant differences in secondary outcome measures, including length of hospitalization, visual analog pain scale scores, cumulative opioid usage, and rate of acute chest syndrome. Inhaled nitric oxide was well tolerated, with no increase in serious adverse events. Increases in venous methemoglobin concentration confirmed adherence and randomization but did not exceed 5% in any study participant. Significant increases in plasma nitrate occurred in the treatment group, but there were no observed increases in plasma or whole blood nitrite.
Conclusion: Among patients with SCD hospitalized with VOC, the use of inhaled nitric oxide compared with placebo did not improve time to crisis resolution.
Trial registration: clinicaltrials.gov Identifier: NCT00094887.
Conflict of interest statement
Conflict of Interest and Financial Disclosure:
Mark T. Gladwin has received research support in the form of a Collaborative Research and Development Agreement between the US Government and Ikaria/INO Therapeutics and is listed as a co-inventor on a US Government Patent for the use of nitrite salts for cardiovascular indications. Dr. Gladwin receives grant support from the Institute of Transfusion Medicine, the Hemophilia Center of Western Pennsylvania and Federal funding by the NHLBI, NIDDK and NIAMS of the National Institutes of health (NIH grants R01HL098032, RO1HL096973, RC1DK085852, P30AR058910).
Gregory J. Kato has received research grant support from a cooperative research and development agreement between the National Institutes of Health and Ikaria INO Therapeutics and from the Division of Intramural Research of the National Institutes of Health. Victor R. Gordeuk has received research support from Biomarin and TRF-Pharma, and Emmaus Pharmaceuticals.
Lakshmanan Krishnamurti has received research support from the National Heart Lung and Blood Institute (NHLBI HB-06-06).
James F. Casella has received research support from the National Institutes of Health, NHLBI, NINDS, NCRR, MCHB, Maryland DHMH. Dr. Casella received past research support from Cytrex Corporation for the investigation of a drug (Poloxamer 188) for use in vaso-occlusive crisis. The results of these studies have been reported (ref). No ongoing relationship exists.
James Baldassarre is an employee of Ikaria.
Debra Weiner, MD, PhD has received research support from the FDA Orphan Product Development Grant Program.
Onyinye Onyekwere, MD, has received research support from Novartis Pharmaceuticals, Eli Lilly and Company, Icagen/McNeil, NIH/NHLBI and Anthera Pharmaceuticals, Inc
Carlton Dampier MD received research grant support during the period of this study from Icagen, Inc, BioMarin Pharmaceuticals, Inc, and the National Institutes of Health (U54HL070585, U10HL083705, R21HD049244, N01HB07159). Dr Dampier also had consulting relationships with Anthera Pharmaceuticals Inc, GlycoMimetics Inc, and Wyeth Pharmaceuticals Inc
Lewis Hsu, MD, R. Ward Hagar, MD, Thomas Howard, MD, Brigitta G. Mueller, MD, Rachelle Nuss, MD, Maureen Okam, MD, Lakshmanan Krishnamurti, MD; Brian Berman, M.D., Oswaldo Castro, MD, Victor R. Gordeuk, MD,; Wynona Coles, RT, Mariana Hildesheim, MS, Mary K. Hall, CIP, William C. Blackwelder, PhD, James Baldassarre, MD, Deborah Weiner, MD, James Casella, MD, Carlton Dampier, MD report no relevant conflict of interest related to the participant of this manuscript.
Figures





References
-
- Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003 May 15;101(10):3953–3959. - PubMed
-
- Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96(7):2451–2459. - PubMed
-
- Belcher JD, Mahaseth H, Welch TE, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005 Jun;288(6):H2715–2725. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

