An important role of lymphatic vessel activation in limiting acute inflammation
- PMID: 21364190
- PMCID: PMC3099581
- DOI: 10.1182/blood-2010-10-316356
An important role of lymphatic vessel activation in limiting acute inflammation
Abstract
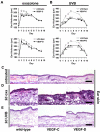
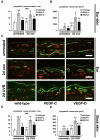
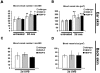
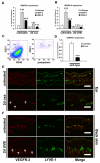
In contrast to the established role of blood vessel remodeling in inflammation, the biologic function of the lymphatic vasculature in acute inflammation has remained less explored. We studied 2 established models of acute cutaneous inflammation, namely, oxazolone-induced delayed-type hypersensitivity reactions and ultraviolet B irradiation, in keratin 14-vascular endothelial growth factor (VEGF)-C and keratin 14-VEGF-D transgenic mice. These mice have an expanded network of cutaneous lymphatic vessels. Transgenic delivery of the lymphangiogenic factors VEGF-C and the VEGFR-3 specific ligand mouse VEGF-D significantly limited acute skin inflammation in both experimental models, with a strong reduction of dermal edema. Expression of VEGFR-3 by lymphatic endothelium was strongly down-regulated at the mRNA and protein level in acutely inflamed skin, and no VEGFR-3 expression was detectable on inflamed blood vessels and dermal macrophages. There was no major change of the inflammatory cell infiltrate or the composition of the inflammatory cytokine milieu in the inflamed skin of VEGF-C or VEGF-D transgenic mice. However, the increased network of lymphatic vessels in these mice significantly enhanced lymphatic drainage from the ear skin. These results provide evidence that specific lymphatic vessel activation limits acute skin inflammation via promotion of lymph flow from the skin and reduction of edema formation.
Figures


References
-
- Halin C, Detmar M. Inflammation, angiogenesis, and lymphangiogenesis. Methods Enzymol. 2008;445:1–25. - PubMed
-
- Bainbridge J, Sivakumar B, Paleolog E. Angiogenesis as a therapeutic target in arthritis: lessons from oncology. Curr Pharm Des. 2006;12(21):2631–2644. - PubMed
-
- Danese S, Sans M, de la Motte C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130(7):2060–2073. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

