PML-RARA can increase hematopoietic self-renewal without causing a myeloproliferative disease in mice
- PMID: 21364283
- PMCID: PMC3068978
- DOI: 10.1172/JCI42953
PML-RARA can increase hematopoietic self-renewal without causing a myeloproliferative disease in mice
Abstract
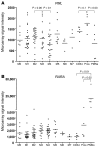
Acute promyelocytic leukemia (APL) is characterized by the t(15;17) translocation that generates the fusion protein promyelocytic leukemia-retinoic acid receptor α (PML-RARA) in nearly all cases. Multiple prior mouse models of APL constitutively express PML-RARA from a variety of non-Pml loci. Typically, all animals develop a myeloproliferative disease, followed by leukemia in a subset of animals after a long latent period. In contrast, human APL is not associated with an antecedent stage of myeloproliferation. To address this discrepancy, we have generated a system whereby PML-RARA expression is somatically acquired from the mouse Pml locus in the context of Pml haploinsufficiency. We found that physiologic PML-RARA expression was sufficient to direct a hematopoietic progenitor self-renewal program in vitro and in vivo. However, this expansion was not associated with evidence of myeloproliferation, more accurately reflecting the clinical presentation of human APL. Thus, at physiologic doses, PML-RARA primarily acts to increase hematopoietic progenitor self-renewal, expanding a population of cells that are susceptible to acquiring secondary mutations that cause progression to leukemia. This mouse model provides a platform for more accurately dissecting the early events in APL pathogenesis.
Figures





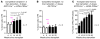
References
-
- Kogan SC. Mouse models of acute promyelocytic leukemia. Curr Top Microbiol Immunol. 2007;313:3–29. - PubMed
-
- Westervelt P, Ley TJ. Seed versus soil: the importance of the target cell for transgenic models of human leukemias. Blood. 1999;93(7):2143–2148. - PubMed
-
- Grignani F, et al. PML/RAR alpha fusion protein expression in normal human hematopoietic progenitors dictates myeloid commitment and the promyelocytic phenotype. Blood. 2000;96(4):1531–1537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

