Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions
- PMID: 21364530
- PMCID: PMC3094119
- DOI: 10.1038/emboj.2011.53
Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions
Abstract
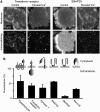
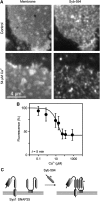
Membrane proteins and membrane lipids are frequently organized in submicron-sized domains within cellular membranes. Factors thought to be responsible for domain formation include lipid-lipid interactions, lipid-protein interactions and protein-protein interactions. However, it is unclear whether the domain structure is regulated by other factors such as divalent cations. Here, we have examined in native plasma membranes and intact cells the role of the second messenger Ca(2+) in membrane protein organization. We find that Ca(2+) at low micromolar concentrations directly redistributes a structurally diverse array of membrane proteins via electrostatic effects. Redistribution results in a more clustered pattern, can be rapid and triggered by Ca(2+) influx through voltage-gated calcium channels and is reversible. In summary, the data demonstrate that the second messenger Ca(2+) strongly influences the organization of membrane proteins, thus adding a novel and unexpected factor that may control the domain structure of biological membranes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

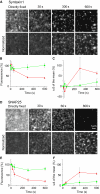
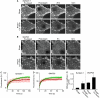
 , which yields the maximal recovery (maxrec) and the half-time of recovery (t1/2), was fitted to the averaged traces obtained from one individual experiment. Before fitting, the traces were rescaled setting the pre-bleach and the post-bleach values to 100 and 0%, respectively. Averaged fits are shown in Supplementary Figure S6 and as black lines with adjusted scaling in (C, D) for comparison to averaged raw data. From the individual fits maximal recovery and half-time of recovery were further used for calculation of the immobile fraction (immobile fraction=100% − maximal recovery) and the apparent lateral diffusion coefficient. The size of the immobile pools increased after rising Ca2+ from 15 to 43% for syntaxin and from 23 to 49% for SNAP25. Values are given as mean±s.e.m. (n=3). (E) Ca2+-induced change of the apparent lateral diffusion coefficient. Values are given as mean±s.e.m. (n=3).
, which yields the maximal recovery (maxrec) and the half-time of recovery (t1/2), was fitted to the averaged traces obtained from one individual experiment. Before fitting, the traces were rescaled setting the pre-bleach and the post-bleach values to 100 and 0%, respectively. Averaged fits are shown in Supplementary Figure S6 and as black lines with adjusted scaling in (C, D) for comparison to averaged raw data. From the individual fits maximal recovery and half-time of recovery were further used for calculation of the immobile fraction (immobile fraction=100% − maximal recovery) and the apparent lateral diffusion coefficient. The size of the immobile pools increased after rising Ca2+ from 15 to 43% for syntaxin and from 23 to 49% for SNAP25. Values are given as mean±s.e.m. (n=3). (E) Ca2+-induced change of the apparent lateral diffusion coefficient. Values are given as mean±s.e.m. (n=3).
References
-
- Barnstable CJ, Hofstein R, Akagawa K (1985) A marker of early amacrine cell development in rat retina. Brain Res 352: 286–290 - PubMed
-
- Bollmann JH, Sakmann B (2005) Control of synaptic strength, timing by the release-site Ca2+ signal. Nat Neurosci 8: 426–434 - PubMed
-
- Bolte S, Cordelieres FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

