Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells
- PMID: 21364643
- PMCID: PMC3032304
- DOI: 10.1038/cddis.2010.14
Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells
Abstract
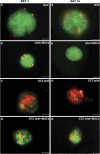
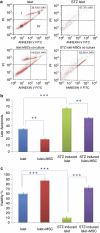
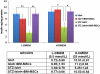
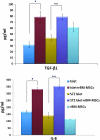
The maintenance of viable and functional islets is critical in successful pancreatic islet transplantation from cadaveric sources. During the isolation procedure, islets are exposed to a number of insults including ischemia, oxidative stress and cytokine injury that cause a reduction in the recovered viable islet mass. A novel approach was designed in which streptozotocin (STZ)-damaged rat pancreatic islets (rPIs) were indirectly cocultured with rat bone marrow-derived mesenchymal stem cells (rBM-MSCs) to maintain survival of the cultured rPIs. The results indicated that islets cocultured with rBM-MSCs secreted an increased level of insulin after 14 days, whereas non-cocultured islets gradually deteriorated and cell death occurred. The cocultivation of rBM-MSCs with islets and STZ-damaged islets showed the expression of IL6 and transforming growth factor-β1 in the culture medium, besides the expression of the antiapoptotic genes (Mapkapk2, Tnip1 and Bcl3), implying the cytoprotective, anti-inflammatory and antiapoptotic effects of rBM-SCs through paracrine actions.
Figures


References
-
- Ricordi C. Islet transplantation: a brave new world. Diabetes. 2003;52:1595–1603. - PubMed
-
- Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20:270–276. - PubMed
-
- Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, Khan A, et al. Improved human islet isolation using nicotinamide. Am J Transplant. 2006;6:2060–2068. - PubMed
-
- Rabinovitch A, Russell T, Mintz DH. Factors from fibroblasts promote pancreatic islet B cell survival in tissue culture. Diabetes. 1979;28:1108–1113. - PubMed
-
- Yang Z, Chen M, Carter JD, Ellett JD, Smith KM, Nadler JL. Inflammation blockade improves pancreatic islet function. Transplant Proc. 2004;36:2864–2865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

